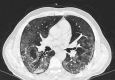
Severe COVID-19 in a patient with chronic graft-versus-host disease after hematopoietic stem cell transplant successfully treated with ruxolitinib
- PMID: 32629531
- PMCID: PMC7361240
- DOI: 10.1111/tid.13401
Severe COVID-19 in a patient with chronic graft-versus-host disease after hematopoietic stem cell transplant successfully treated with ruxolitinib
Abstract
Graft-versus-host disease (GVHD) is a common complication of hematopoietic stem cell transplant, which is known to be mediated by cytotoxic T-cell effectors and dysregulated inflammatory cytokines. Similarly, the lung injury observed in severe COVID-19 cases appears to be related to a massive production of pro-inflammatory cytokines. The selective JAK1/2 inhibitor ruxolitinib has shown promising results in the context of GVHD, and different trials are currently underway in patients with severe COVID-19; nevertheless, no clinical observation of safety or efficacy of treatment with ruxolitinib in this context has been published yet. We describe a first case of severe COVID-19 developed after hematopoietic stem cell transplantation in a patient with a concomitant chronic GVHD (cGVHD), in which a treatment with ruxolitinib was administered with good tolerance and positive outcome.
Keywords: ARDS (acute respiratory distress syndrome); COVID-19; chronic graft-versus-host disease (cGVHD); hematopoietic stem cell transplant (SCT); ruxolitinib.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Olivieri A received honoraria as consultant from Novartis in 2017 and 2018. The other authors declare no conflict of interest.
Figures
References
-
- https://www.fda.gov/media/134922/download. Accessed June 24, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous