A Structurally Simple Vaccine Candidate Reduces Progression and Dissemination of Triple-Negative Breast Cancer
- PMID: 32629615
- PMCID: PMC7322362
- DOI: 10.1016/j.isci.2020.101250
A Structurally Simple Vaccine Candidate Reduces Progression and Dissemination of Triple-Negative Breast Cancer
Abstract
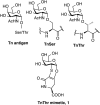
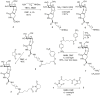
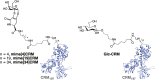
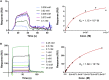
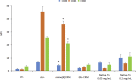
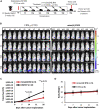
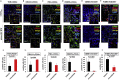
The Tn antigen is a well-known tumor-associated carbohydrate determinant, often incorporated in glycopeptides to develop cancer vaccines. Herein, four copies of a conformationally constrained mimetic of the antigen TnThr (GalNAc-Thr) were conjugated to the adjuvant CRM197, a protein licensed for human use. The resulting vaccine candidate, mime[4]CRM elicited a robust immune response in a triple-negative breast cancer mouse model, correlated with high frequency of CD4+ T cells and low frequency of M2-type macrophages, which reduces tumor progression and lung metastasis growth. Mime[4]CRM-mediated activation of human dendritic cells is reported, and the proliferation of mime[4]CRM-specific T cells, in cancer tissue and peripheral blood of patients with breast cancer, is demonstrated. The locked conformation of the TnThr mimetic and a proper presentation on the surface of CRM197 may explain the binding of the conjugate to the anti-Tn antibody Tn218 and its efficacy to fight cancer cells in mice.
Keywords: Cancer; Immunology; Medical Biochemistry.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests F.B. is an employee of the GSK group of companies. All the other authors declare no competing financial interests.
Figures



References
-
- Amedei A., Niccolai E., Benagiano M., Della Bella C., Cianchi F., Bechi P., Taddei A., Bencini L., Farsi M., Cappello P. Ex vivo analysis of pancreatic cancer-infiltrating T lymphocytes reveals that ENO-specific Tregs accumulate in tumor tissue and inhibit Th1/Th17 effector cell functions. Cancer Immunol. Immunother. 2013;62:1249–1260. - PMC - PubMed
-
- Ardá A., Bosco R., Sastre J., Cañada F.J., André S., Gabius H.-J., Richichi B., Jiménez-Barbero J., Nativi C. Structural insights into the binding of sugar receptors (lectins) to a synthetic tricyclic tn mimetic and its glycopeptide version. Eur. J. Org. Chem. 2015;2015:6823–6831.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

