Sugary Logistics Gone Wrong: Membrane Trafficking and Congenital Disorders of Glycosylation
- PMID: 32629928
- PMCID: PMC7369703
- DOI: 10.3390/ijms21134654
Sugary Logistics Gone Wrong: Membrane Trafficking and Congenital Disorders of Glycosylation
Abstract
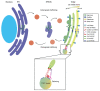
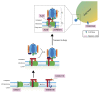
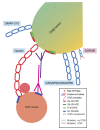
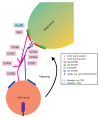
Glycosylation is an important post-translational modification for both intracellular and secreted proteins. For glycosylation to occur, cargo must be transported after synthesis through the different compartments of the Golgi apparatus where distinct monosaccharides are sequentially bound and trimmed, resulting in increasingly complex branched glycan structures. Of utmost importance for this process is the intraorganellar environment of the Golgi. Each Golgi compartment has a distinct pH, which is maintained by the vacuolar H+-ATPase (V-ATPase). Moreover, tethering factors such as Golgins and the conserved oligomeric Golgi (COG) complex, in concert with coatomer (COPI) and soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-mediated membrane fusion, efficiently deliver glycosylation enzymes to the right Golgi compartment. Together, these factors maintain intra-Golgi trafficking of proteins involved in glycosylation and thereby enable proper glycosylation. However, pathogenic mutations in these factors can cause defective glycosylation and lead to diseases with a wide variety of symptoms such as liver dysfunction and skin and bone disorders. Collectively, this group of disorders is known as congenital disorders of glycosylation (CDG). Recent technological advances have enabled the robust identification of novel CDGs related to membrane trafficking components. In this review, we highlight differences and similarities between membrane trafficking-related CDGs.
Keywords: Golgi apparatus; congenital disorders of glycosylation; glycosylation; membrane trafficking; post-translational modification; secretory pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

