Transcriptomic Analysis of Gill and Kidney from Asian Seabass (Lates calcarifer) Acclimated to Different Salinities Reveals Pathways Involved with Euryhalinity
- PMID: 32630108
- PMCID: PMC7397140
- DOI: 10.3390/genes11070733
Transcriptomic Analysis of Gill and Kidney from Asian Seabass (Lates calcarifer) Acclimated to Different Salinities Reveals Pathways Involved with Euryhalinity
Abstract
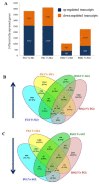
Asian seabass (or commonly known as barramundi), Lates calcarifer, is a bony euryhaline teleost from the Family Latidae, inhabiting nearshore, estuarine, and marine connected freshwaters throughout the tropical Indo-West Pacific region. The species is catadromous, whereby adults spawn in salinities between 28 and 34 ppt at the mouth of estuaries, with resultant juveniles usually moving into brackish and freshwater systems to mature, before returning to the sea to spawn again as adults. The species lives in both marine and freshwater habitats and can move quickly between the two; thus, the species' ability to tolerate changes in salinity makes it a good candidate for studying the salinity acclimation response in teleosts. In this study, the transcriptome of two major osmoregulatory organs (gills and kidneys) of young juvenile Asian seabass reared in freshwater and seawater were compared. The euryhaline nature of Asian seabass was found to be highly pliable and the moldability of the trait was further confirmed by histological analyses of gills and kidneys. Differences in major expression pathways were observed, with differentially expressed genes including those related to osmoregulation, tissue/organ morphogenesis, and cell volume regulation as central to the osmo-adaptive response. Additionally, genes coding for mucins were upregulated specifically under saline conditions, whereas several genes important for growth and development, as well as circadian entrainment were specifically enriched in fish reared in freshwater. Routing of the circadian rhythm mediated by salinity changes could be the initial step in salinity acclimation and possibly migration in euryhaline fish species such as the Asian seabass.
Keywords: Asian seabass; acclimation; euryhalinity; fish; freshwater transcriptome; marine transcriptome; osmoregulation; pliable trait; transcriptome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Differential transcriptomic analyses revealed genes and signaling pathways involved in iono-osmoregulation and cellular remodeling in the gills of euryhaline Mozambique tilapia, Oreochromis mossambicus.BMC Genomics. 2014 Oct 23;15(1):921. doi: 10.1186/1471-2164-15-921. BMC Genomics. 2014. PMID: 25342237 Free PMC article.
-
Structure and gene expression changes of the gill and liver in juvenile black porgy (Acanthopagrus schlegelii) under different salinities.Comp Biochem Physiol Part D Genomics Proteomics. 2024 Jun;50:101228. doi: 10.1016/j.cbd.2024.101228. Epub 2024 Mar 20. Comp Biochem Physiol Part D Genomics Proteomics. 2024. PMID: 38547756
-
Evidence for a role of arginine vasotocin receptors in the gill during salinity acclimation by a euryhaline teleost fish.Am J Physiol Regul Integr Comp Physiol. 2019 Jun 1;316(6):R735-R750. doi: 10.1152/ajpregu.00328.2018. Epub 2019 Mar 27. Am J Physiol Regul Integr Comp Physiol. 2019. PMID: 30916577
-
Endocrine and osmoregulatory responses to tidally-changing salinities in fishes.Gen Comp Endocrinol. 2022 Sep 15;326:114071. doi: 10.1016/j.ygcen.2022.114071. Epub 2022 Jun 11. Gen Comp Endocrinol. 2022. PMID: 35697315 Review.
-
Osmotic control in marine animals.Symp Soc Exp Biol. 1985;39:207-44. Symp Soc Exp Biol. 1985. PMID: 2939580 Review.
Cited by
-
Freshwater transfer affected intestinal microbiota with correlation to cytokine gene expression in Asian sea bass.Front Microbiol. 2023 Apr 6;14:1097954. doi: 10.3389/fmicb.2023.1097954. eCollection 2023. Front Microbiol. 2023. PMID: 37089546 Free PMC article.
-
Scale Drop Disease Virus (SDDV) and Lates calcarifer Herpes Virus (LCHV) Coinfection Downregulate Immune-Relevant Pathways and Cause Splenic and Kidney Necrosis in Barramundi Under Commercial Farming Conditions.Front Genet. 2021 Jun 18;12:666897. doi: 10.3389/fgene.2021.666897. eCollection 2021. Front Genet. 2021. PMID: 34220943 Free PMC article.
-
Comparative Nutritional and Histological Analysis of Malabar Red Snapper (Lutjanus malabaricus) and Asian Seabass (Lates calcarifer).Animals (Basel). 2024 Jun 17;14(12):1803. doi: 10.3390/ani14121803. Animals (Basel). 2024. PMID: 38929422 Free PMC article.
-
Transcriptomic and Proteomic Analysis of Marine Nematode Litoditis marina Acclimated to Different Salinities.Genes (Basel). 2022 Apr 7;13(4):651. doi: 10.3390/genes13040651. Genes (Basel). 2022. PMID: 35456458 Free PMC article.
-
Field application of de novo transcriptomic analysis to evaluate the effects of sublethal freshwater salinization on Gasterosteus aculeatus in urban streams.PLoS One. 2024 Mar 13;19(3):e0298213. doi: 10.1371/journal.pone.0298213. eCollection 2024. PLoS One. 2024. PMID: 38478568 Free PMC article.
References
-
- Froese R., Pauly D. FishBase. World Wide Web Electronic Publication. [(accessed on 27 March 2016)]; Available online: www.fishbase.org.
-
- Schultz E.T., McCormick D.S. Euryhalinity in an evolutionary context. Fish Physiol. 2012;32:477–533.
-
- Kirsch R., Humbert W., Rodeau J.L. Control of the blood osmolarity in fishes with references to the functional anatomy of the gut. In: Pequeux A., Gilles R., Bolis L., editors. Osmoregulation in Estuarine and Marine Animals: Proceedings of the Invited Lectures to a Symposium Organized within the 5th Conference of the European Society for Comparative Physiology and Biochemistry—Taormina, Sicily, Italy, 5–8 September 1983. Springer; Berlin/Heidelberg, Germany: 1984.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

