Wnt/β-Catenin Signaling in Oral Carcinogenesis
- PMID: 32630122
- PMCID: PMC7369957
- DOI: 10.3390/ijms21134682
Wnt/β-Catenin Signaling in Oral Carcinogenesis
Abstract
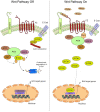
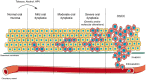
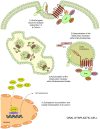
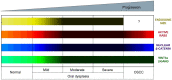
Oral carcinogenesis is a complex and multifactorial process that involves cumulative genetic and molecular alterations, leading to uncontrolled cell proliferation, impaired DNA repair and defective cell death. At the early stages, the onset of potentially malignant lesions in the oral mucosa, or oral dysplasia, is associated with higher rates of malignant progression towards carcinoma in situ and invasive carcinoma. Efforts have been made to get insights about signaling pathways that are deregulated in oral dysplasia, as these could be translated into novel markers and might represent promising therapeutic targets. In this context, recent evidence underscored the relevance of the Wnt/β-catenin signaling pathway in oral dysplasia, as this pathway is progressively "switched on" through the different grades of dysplasia (mild, moderate and severe dysplasia), with the consequent nuclear translocation of β-catenin and expression of target genes associated with the maintenance of representative traits of oral dysplasia, namely cell proliferation and viability. Intriguingly, recent studies provide an unanticipated connection between active β-catenin signaling and deregulated endosome trafficking in oral dysplasia, highlighting the relevance of endocytic components in oral carcinogenesis. This review summarizes evidence about the role of the Wnt/β-catenin signaling pathway and the underlying mechanisms that account for its aberrant activation in oral carcinogenesis.
Keywords: Rab5; destruction complex; dysplasia; endosome; oral cancer; β-catenin.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

