The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis
- PMID: 32630462
- PMCID: PMC7350249
- DOI: 10.3390/ijms21124520
The Presence of Seminal Plasma during Liquid Storage of Pig Spermatozoa at 17 °C Modulates Their Ability to Elicit In Vitro Capacitation and Trigger Acrosomal Exocytosis
Abstract
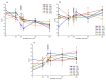
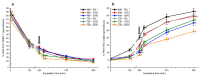
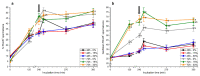
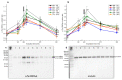
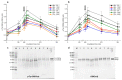
Although seminal plasma is essential to maintain sperm integrity and function, it is diluted/removed prior to liquid storage and cryopreservation in most mammalian species. This study sought to evaluate, using the pig as a model, whether storing semen in the presence of seminal plasma affects the sperm ability to elicit in vitro capacitation and acrosomal exocytosis. Upon collection, seminal plasma was separated from sperm samples, which were diluted in a commercial extender, added with seminal plasma (15% or 30%), and stored at 17 °C for 48 or 72 h. Sperm cells were subsequently exposed to capacitating medium for 4 h, and then added with progesterone to induce acrosomal exocytosis. Sperm motility, acrosome integrity, membrane lipid disorder, intracellular Ca2+ levels, mitochondrial activity, and tyrosine phosphorylation levels of glycogen synthase kinase-3 (GSK3)α/β were determined after 0, 2, and 4 h of incubation, and after 5, 30, and 60 min of progesterone addition. Results showed that storing sperm at 17 °C with 15% or 30% seminal plasma led to reduced percentages of viable spermatozoa exhibiting an exocytosed acrosome, mitochondrial membrane potential, intracellular Ca2+ levels stained by Fluo3, and tyrosine phosphorylation levels of GSK3α/β after in vitro capacitation and progesterone-induced acrosomal exocytosis. Therefore, the direct contact between spermatozoa and seminal plasma during liquid storage at 17 °C modulated their ability to elicit in vitro capacitation and undergo acrosomal exocytosis, via signal transduction pathways involving Ca2+ and Tyr phosphorylation of GSK3α/β. Further research is required to address whether such a modulating effect has any impact upon sperm fertilizing ability.
Keywords: acrosomal exocytosis; in vitro capacitation; seminal plasma; spermatozoa.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported herein.
Figures




References
-
- Barranco I., Padilla L., Parrilla I., Álvarez-Barrientos A., Pérez-Patiño C., Peña F.J., Martínez E.A., Rodriguez-Martínez H., Roca J. Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles. Sci. Rep. 2019;9:11584. doi: 10.1038/s41598-019-48095-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

