New Variants of Nitroxide Mediated Polymerization
- PMID: 32630664
- PMCID: PMC7408045
- DOI: 10.3390/polym12071481
New Variants of Nitroxide Mediated Polymerization
Abstract
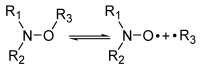
Nitroxide-mediated polymerization is now a mature technique, at 35 years of age. During this time, several variants have been developed: electron spin capture polymerization (ESCP), photoNMP (NMP2), chemically initiated NMP (CI-NMP), spin label NMP (SL-NMP), and plasmon-initiated NMP (PI-NMP). This mini-review is devoted to the features and applications of these variants.
Keywords: CI-NMP; ESCP; NMP2; PI-NMP; SLNMP; nitroxide mediated polymerization.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the writing of the manuscript and in the decision to publish the results.
Figures

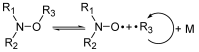











References
-
- Jones L.W., Major R.T. Substituted O-alkyl hydroxylamines chemically related to medicinally valuable amines. J. Am. Chem. Soc. 1927;49:1527–1540. doi: 10.1021/ja01405a021. - DOI
-
- Kovtun G.A., Aleksandrov A.L., Golubev V.A. Interaction of Peroxide Radicals with Esters of Hydroxylamines. Russ. Chem. Bull. 1974;23:2115–2121. doi: 10.1007/BF00921266. Izv. Akad. Nauk SSSR, Ser. Khim.1974, 2197–2203. - DOI
-
- Solomon D.H., Rizzardo E., Cacioli P. Polymerization Process and Polymers Produced Thereby. 4,581,429. U.S. Patent. 1986 Aug 4
-
- Matyjaszewski K. Controlled Radical Polymerization. Current Opin. Solid State Materials Sci. 1996;1996. 1:769–776. doi: 10.1016/S1359-0286(96)80101-X. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

