Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China
- PMID: 32631393
- PMCID: PMC7336107
- DOI: 10.1186/s13054-020-03098-9
Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China
Abstract
Background: The global numbers of confirmed cases and deceased critically ill patients with COVID-19 are increasing. However, the clinical course, and the 60-day mortality and its predictors in critically ill patients have not been fully elucidated. The aim of this study is to identify the clinical course, and 60-day mortality and its predictors in critically ill patients with COVID-19.
Methods: Critically ill adult patients admitted to intensive care units (ICUs) from 3 hospitals in Wuhan, China, were included. Data on demographic information, preexisting comorbidities, laboratory findings at ICU admission, treatments, clinical outcomes, and results of SARS-CoV-2 RNA tests and of serum SARS-CoV-2 IgM were collected including the duration between symptom onset and negative conversion of SARS-CoV-2 RNA.
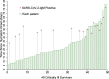
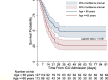
Results: Of 1748 patients with COVID-19, 239 (13.7%) critically ill patients were included. Complications included acute respiratory distress syndrome (ARDS) in 164 (68.6%) patients, coagulopathy in 150 (62.7%) patients, acute cardiac injury in 103 (43.1%) patients, and acute kidney injury (AKI) in 119 (49.8%) patients, which occurred 15.5 days, 17 days, 18.5 days, and 19 days after the symptom onset, respectively. The median duration of the negative conversion of SARS-CoV-2 RNA was 30 (range 6-81) days in 49 critically ill survivors that were identified. A total of 147 (61.5%) patients deceased by 60 days after ICU admission. The median duration between ICU admission and decease was 12 (range 3-36). Cox proportional-hazards regression analysis revealed that age older than 65 years, thrombocytopenia at ICU admission, ARDS, and AKI independently predicted the 60-day mortality.
Conclusions: Severe complications are common and the 60-day mortality of critically ill patients with COVID-19 is considerably high. The duration of the negative conversion of SARS-CoV-2 RNA and its association with the severity of critically ill patients with COVID-19 should be seriously considered and further studied.
Keywords: Acute kidney injury; Acute respiratory syndrome; COVID-19; Mortality; Thrombocytopenia.
Conflict of interest statement
All the authors state that there are no conflicts of interest related to this study.
Figures

References
-
- Coronavirus disease 2019 (COVID-19) Situation Report-140. Published June 8, 2020. Accessed June 8, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

