Systematic review of the registered clinical trials for coronavirus disease 2019 (COVID-19)
- PMID: 32631442
- PMCID: PMC7338108
- DOI: 10.1186/s12967-020-02442-5
Systematic review of the registered clinical trials for coronavirus disease 2019 (COVID-19)
Abstract
Background: Since the outbreak of coronavirus disease 2019 (COVID-19), many researchers in China have performed related clinical research. However, systematic reviews of the registered clinical trials are still lacking. Therefore, we conducted a systematic review of clinical trials for COVID-19 to summarize their characteristics.
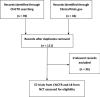
Methods: This study is based on the PRISMA recommendations in the Cochrane handbook. The Chinese Clinical Registration Center and the ClinicalTrials.gov databases were searched to identify registered clinical trials related to COVID-19. The retrieval inception date was February 9, 2020. Two researchers independently selected the literature based on the inclusion and exclusion criteria, extracted data, and evaluated the risk of bias.
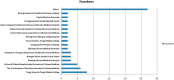
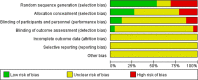
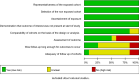
Results: A total of 75 registered clinical trials (63 interventional studies and 12 observational studies) for COVID-19 were identified. The majority of clinical trials were sponsored by Chinese hospitals. Only 11 trials have begun to recruit patients, and none of the registered clinical trials have been completed; 34 trials were early clinical exploratory trials or in the pre-experiment stage, 13 trials were phase III, and four trials were phase IV. The intervention methods included traditional Chinese medicine in 26 trials, Western medicine in 30 trials, and integrated traditional Chinese medicine and Western medicine in 19 trials. The subjects were primarily non-critical adult patients (≥ 18 years old). The median sample size of the trials was 100 (IQR: 60-200), and the median length of the trial periods was 179 d (IQR: 94-366 d). The main outcomes were clinical observation and examinations. Overall, the methodological quality of both the interventional trials and observational studies was low.
Conclusions: Intensive clinical trials on the treatment of COVID-19 using traditional Chinese medicine and Western medicine are ongoing or will be performed in China. However, based on the uncertain methodological quality, small sample size, and long trial duration, we will not be able to obtain reliable, high-quality clinical evidence regarding the treatment of COVID-19 in the near future. Improving the quality of study design, prioritizing promising drugs, and using different designs and statistical methods are worth advocating and recommending for clinical trials of COVID-19 in the future.
Keywords: 2019-nCoV; COVID-19; Interventional trial; New coronavirus pneumonia; Observational study; Registered clinical trial; Systematic review.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical