Population Pharmacokinetics of Praziquantel in Pregnant and Lactating Filipino Women Infected with Schistosoma japonicum
- PMID: 32631820
- PMCID: PMC7449211
- DOI: 10.1128/AAC.00566-20
Population Pharmacokinetics of Praziquantel in Pregnant and Lactating Filipino Women Infected with Schistosoma japonicum
Abstract
An estimated 40 million women of reproductive age are infected with one of three species of the waterborne parasite Schistosoma spp. Treatment with praziquantel (PZQ) via mass drug administration (MDA) campaigns is the mainstay of schistosomiasis control for populations living in areas of endemicity. The World Health Organization recommends that pregnant and lactating women be included in schistosomiasis MDA programs, and several recent studies have evaluated the safety and efficacy of PZQ use during pregnancy. To date, there are no data describing PZQ pharmacokinetics (PK) during pregnancy or among lactating postpartum women. As part of a randomized controlled trial investigating the safety and efficacy of PZQ during human pregnancy, we examined the PK of this therapeutic drug among three distinct cohorts of women infected with S. japonicum in Leyte, Philippines. Specifically, we studied the PK properties of PZQ among early- and late-gestation pregnant women (n = 15 each) and lactating postpartum women (n = 15) with schistosomiasis. We found that women in early pregnancy had increased apparent clearance and lower area-under-the-curve (AUC0-24) values that may be related to physiological changes in drug clearance and/or changes in oral bioavailability. There was no relationship between body weight and apparent clearance. The mean ± standard deviation partition ratio of plasma to breast milk was 0.36. ± 0.13. The estimated median infant PZQ daily dose would be 0.037 mg/kg of body weight ingested from breast milk, which is significantly lower than the dosage required for antischistosomal activity and not known to be harmful to the infant. Our PK data do not support the suggestion to delay breastfeeding 72 h after taking PZQ. Results can help inform future drug efficacy studies in pregnant and lactating women with schistosomiasis.
Keywords: PK; Schistosoma japonicum; breast milk; lactation; pharmacokinetics; praziquantel; pregnancy; schistosomiasis.
Copyright © 2020 Bustinduy et al.
Figures
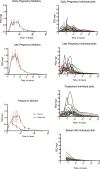
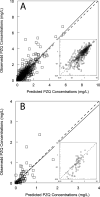
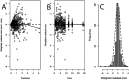



References
-
- WHO. 2016. Schistosomiasis: number of people treated worldwide in 2014. Wkly Epidemiol Rec 5:53–60. - PubMed
-
- GBD 2015 DALYs and HALE Collaborators. 2016. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1603–1658. doi: 10.1016/S0140-6736(16)31460-X. - DOI - PMC - PubMed
-
- WHO. 2006. Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions. WHO, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

