3-Hydroxybutyrate Derived from Poly-3-Hydroxybutyrate Mobilization Alleviates Protein Aggregation in Heat-Stressed Herbaspirillum seropedicae SmR1
- PMID: 32631857
- PMCID: PMC7440793
- DOI: 10.1128/AEM.01265-20
3-Hydroxybutyrate Derived from Poly-3-Hydroxybutyrate Mobilization Alleviates Protein Aggregation in Heat-Stressed Herbaspirillum seropedicae SmR1
Abstract
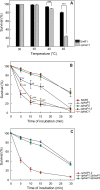
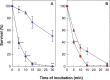
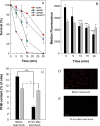
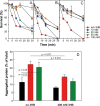
Under conditions of carbon starvation or thermal, osmotic, or oxidative shock, mutants affected in the synthesis or mobilization of poly-3-hydroxybutyrate (PHB) are known to survive less well. It is still unclear if the synthesis and accumulation of PHB are sufficient to protect bacteria against stress conditions or if the stored PHB has to be mobilized. Here, we demonstrated that mobilization of PHB in Herbaspirillum seropedicae SmR1 was heat-shock activated at 45°C. In situ proton (1H) nuclear magnetic resonance spectroscopy (i.e., 1H-nuclear magnetic resonance) showed that heat shock increased amounts of 3-hydroxybutyrate (3HB) only in H. seropedicae strains able to synthesize and mobilize PHB. H. seropedicae SmR1 mutants unable to synthesize or mobilize PHB were more susceptible to heat shock and survived less well than the parental strain. When 100 mM 3-hydroxybutyrate was added to the medium, the ΔphaC1 strain (an H. seropedicae mutant unable to synthesize PHB) and the double mutant with deletion of both phaZ1 and phaZ2 (i.e., ΔphaZ1.2) (unable to mobilize PHB) showed partial rescue of heat adaptability (from 0% survival without 3HB to 40% of the initial viable population). Addition of 200 mM 3HB before the imposition of heat shock reduced protein aggregation to 15% in the ΔphaC1 mutant and 12% in the ΔphaZ1.2 mutant. We conclude that H. seropedicae SmR1 is naturally protected by 3HB released by PHB mobilization, while mutants unable to generate large amounts of 3HB under heat shock conditions are less able to cope with heat damage.IMPORTANCE Bacteria are subject to abrupt changes in environmental conditions affecting their growth, requiring rapid adaptation. Increasing the concentration of some metabolites can protect bacteria from hostile conditions that lead to protein denaturation and precipitation, as well as damage to plasma membranes. In this work, we demonstrated that under thermal shock, the bacterium Herbaspirillum seropedicae depolymerized its intracellular stock polymer known as poly-3-hydroxybutyrate (PHB), rapidly increasing the concentration of 3-hydroxybutyrate (3HB) and decreasing protein precipitation by thermal denaturation. Mutant H. seropedicae strains unable to produce or depolymerize PHB suffered irreparable damage during thermal shock, resulting in fast death when incubated at 45°C. Our results will contribute to the development of bacteria better adapted to high temperatures found either in natural conditions or in industrial processes. In the case of H. seropedicae and other bacteria that interact beneficially with plants, the understanding of PHB metabolism can be decisive for the development of more-competitive strains and their application as biofertilizers in agriculture.
Keywords: 3-hydroxybutyrate; PHA depolymerase; chiral acid R-3-hydroxybutyrate; in situ NMR; phasin; polyhydroxyalkanoate.
Copyright © 2020 American Society for Microbiology.
Figures

References
-
- Mosier AC, Justice NB, Bowen BP, Baran R, Thomas BC, Northen TR, Banfield JF. 2013. Metabolites associated with adaptation of microorganisms to an acidophilic, metal-rich environment identified by stable-isotope-enabled metabolomics. mBio 4:e00484-12. doi: 10.1128/mBio.00484-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

