Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine
- PMID: 32632016
- PMCID: PMC7382259
- DOI: 10.1073/pnas.2002266117
Impaired estrogen signaling underlies regulatory T cell loss-of-function in the chronically inflamed intestine
Abstract
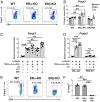
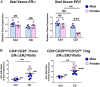
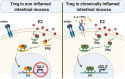
Signaling of 17β-estradiol (estrogen) through its two nuclear receptors, α and β (ERα, ERβ), is an important mechanism of transcriptional regulation. Although ERs are broadly expressed by cells of the immune system, the mechanisms by which they modulate immune responses remain poorly understood. ERβ-specific signaling is reduced in patients with chronic inflammatory diseases, including systemic lupus erythematosus and inflammatory bowel disease, and our previous work suggests that dysregulation of ERβ-specific signaling contributes to enhanced intestinal inflammation in female SAMP/YitFC mice, a spontaneous model of Crohn's disease-like ileitis. The present study builds on these prior observations to identify a nonredundant, immunoprotective role for ERβ-specific signaling in TGF-β-dependent regulatory T cell (Treg) differentiation. Using a strain of congenic SAMP mice engineered to lack global expression of ERβ, we observed dramatic, female-specific exacerbation of intestinal inflammation accompanied by significant reductions in intestinal Treg frequency and function. Impaired Treg suppression in the absence of ERβ was associated with aberrant overexpression of Tsc22d3 (GILZ), a glucocorticoid-responsive transcription factor not normally expressed in mature Tregs, and ex vivo data reveal that forced overexpression of GILZ in mature Tregs inhibits their suppressive function. Collectively, our findings identify a pathway of estrogen-mediated immune regulation in the intestine, whereby homeostatic expression of ERβ normally functions to limit Treg-specific expression of GILZ, thereby maintaining effective immune suppression. Our data suggest that transcriptional cross-talk between glucocorticoid and steroid sex hormone signaling represents an important and understudied regulatory node in chronic inflammatory disease.
Keywords: Crohn’s disease; estrogen; inflammation; inflammatory bowel disease; regulatory T cell.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Klein S. L., Flanagan K. L., Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016). - PubMed
-
- Ortizo R. et al., Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: A meta-analysis of case-controlled and cohort studies. Eur. J. Gastroenterol. Hepatol. 29, 1064–1070 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

