An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment
- PMID: 32632160
- PMCID: PMC7338506
- DOI: 10.1038/s41467-020-17317-y
An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment
Abstract
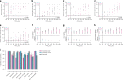
The world is entering a new era of the COVID-19 pandemic in which there is an increasing call for reliable antibody testing. To support decision making on the deployment of serology for either population screening or diagnostics, we present a detailed comparison of serological COVID-19 assays. We show that among the selected assays there is a wide diversity in assay performance in different scenarios and when correlated to virus neutralizing antibodies. The Wantai ELISA detecting total immunoglobulins against the receptor binding domain of SARS CoV-2, has the best overall characteristics to detect functional antibodies in different stages and severity of disease, including the potential to set a cut-off indicating the presence of protective antibodies. The large variety of available serological assays requires proper assay validation before deciding on deployment of assays for specific applications.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Long Q. X., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26, 845–848 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

