Requirements for the differentiation of innate T-bethigh memory-phenotype CD4+ T lymphocytes under steady state
- PMID: 32632165
- PMCID: PMC7338451
- DOI: 10.1038/s41467-020-17136-1
Requirements for the differentiation of innate T-bethigh memory-phenotype CD4+ T lymphocytes under steady state
Erratum in
-
Author Correction: Requirements for the differentiation of innate T-bethigh memory-phenotype CD4+ T lymphocytes under steady state.Nat Commun. 2020 Sep 10;11(1):4624. doi: 10.1038/s41467-020-18568-5. Nat Commun. 2020. PMID: 32913264 Free PMC article.
Abstract
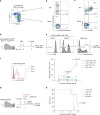
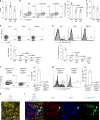
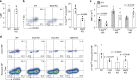
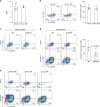
CD4+ T lymphocytes consist of naïve, antigen-specific memory, and memory-phenotype (MP) cell compartments at homeostasis. We recently showed that MP cells exert innate-like effector function during host defense, but whether MP CD4+ T cells are functionally heterogeneous and, if so, what signals specify the differentiation of MP cell subpopulations under homeostatic conditions is still unclear. Here we characterize MP lymphocytes as consisting of T-bethigh, T-betlow, and T-bet- subsets, with innate, Th1-like effector activity exclusively associated with T-bethigh cells. We further show that the latter population depends on IL-12 produced by CD8α+ type 1 dendritic cells (DC1) for its differentiation. Finally, our data demonstrate that this tonic IL-12 production requires TLR-MyD88 signaling independent of foreign agonists, and is further enhanced by CD40-CD40L interactions between DC1 and CD4+ T lymphocytes. We propose that optimal differentiation of T-bethigh MP lymphocytes at homeostasis is driven by self-recognition signals at both the DC and Tcell levels.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

