Microglia phagocytose myelin sheaths to modify developmental myelination
- PMID: 32632287
- PMCID: PMC7483351
- DOI: 10.1038/s41593-020-0654-2
Microglia phagocytose myelin sheaths to modify developmental myelination
Abstract
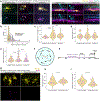
During development, oligodendrocytes contact and wrap neuronal axons with myelin. Similarly to neurons and synapses, excess myelin sheaths are produced and selectively eliminated, but how elimination occurs is unknown. Microglia, the resident immune cells of the central nervous system, engulf surplus neurons and synapses. To determine whether microglia also prune myelin sheaths, we used zebrafish to visualize and manipulate interactions between microglia, oligodendrocytes, and neurons during development. We found that microglia closely associate with oligodendrocytes and specifically phagocytose myelin sheaths. By using a combination of optical, genetic, chemogenetic, and behavioral approaches, we reveal that neuronal activity bidirectionally balances microglial association with neuronal cell bodies and myelin phagocytosis in the optic tectum. Furthermore, multiple strategies to deplete microglia resulted in oligodendrocytes maintaining excessive and ectopic myelin. Our work reveals a neuronal activity-regulated role for microglia in modifying developmental myelin targeting by oligodendrocytes.
Conflict of interest statement
Figures



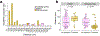







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

