A dual-constriction biological nanopore resolves homonucleotide sequences with high fidelity
- PMID: 32632300
- PMCID: PMC7610451
- DOI: 10.1038/s41587-020-0570-8
A dual-constriction biological nanopore resolves homonucleotide sequences with high fidelity
Abstract
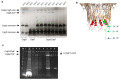
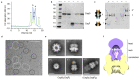
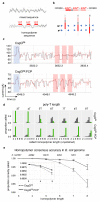
Single-molecule long-read DNA sequencing with biological nanopores is fast and high-throughput but suffers reduced accuracy in homonucleotide stretches. We now combine the CsgG nanopore with the 35-residue N-terminal region of its extracellular interaction partner CsgF to produce a dual-constriction pore with improved signal and base-calling accuracy for homopolymer regions. The electron cryo-microscopy structure of CsgG in complex with full-length CsgF shows that the 33 N-terminal residues of CsgF bind inside the β-barrel of the pore, forming a defined second constriction. In complexes of CsgG bound to a 35-residue CsgF constriction peptide, the second constriction is separated from the primary constriction by ~25 Å. We find that both constrictions contribute to electrical signal modulation during single-stranded DNA translocation. DNA sequencing using a prototype CsgG-CsgF protein pore with two constrictions improved single-read accuracy by 25 to 70% in homopolymers up to 9 nucleotides long.
Conflict of interest statement
VIB and ONT have jointly filed two provisional patent applications on the construction and use of dual constriction pores in nanopore sensing applications (PCT/GB2018/051858 and PCT/GB2018/051191). VIB has a funded research collaboration agreement with VIB related to CsgG-derived nanopores. ONT uses CsgG-derived nanopores in its MinION, GridION and PromethION nanopore sequencing devices. As inventors on VIB IP, SVDV, NVG and HR receive a share in royalty payments. RH, PS, JK, MJ, EJW and LJ are employees of ONT and own company share options.
Figures







Comment in
-
A dual-constriction biological nanopore.Nat Methods. 2020 Sep;17(9):874. doi: 10.1038/s41592-020-0949-y. Nat Methods. 2020. PMID: 32873979 No abstract available.
References
-
- Akeson M, Branton D, Kasianowicz JJ, Brandin E, Deamer DW. Microsecond time-scale discrimination among polycytidylic acid, polyadenylic acid, and polyuridylic acid as homopolymers or as segments within single RNA molecules. Biophysical journal. 1999;77:3227–3233. doi: 10.1016/S0006-3495(99)77153-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

