Tumor dormancy in bone
- PMID: 32632400
- PMCID: PMC7337256
- DOI: 10.1002/cnr2.1156
Tumor dormancy in bone
Abstract
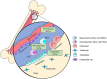
Background: Bone marrow is a common site of metastasis for a number of tumor types, including breast, prostate, and lung cancer, but the mechanisms controlling tumor dormancy in bone are poorly understood. In breast cancer, while advances in drug development, screening practices, and surgical techniques have dramatically improved survival rates in recent decades, metastatic recurrence in the bone remains common and can develop years or decades after elimination of the primary tumor.
Recent findings: It is now understood that tumor cells disseminate to distant metastatic sites at early stages of tumor progression, leaving cancer survivors at a high risk of recurrence. This review will discuss mechanisms of bone lesion development and current theories of how dormant cancer cells behave in bone, as well as a number of processes suspected to be involved in the maintenance of and exit from dormancy in the bone microenvironment.
Conclusions: The bone is a complex microenvironment with a multitude of cell types and processes. Many of these factors, including angiogenesis, immune surveillance, and hypoxia, are thought to regulate tumor cell entry and exit from dormancy in different bone marrow niches.
Keywords: angiogenesis; bone marrow; dormancy; hypoxia; immune surveillance; metastasis.
Conflict of interest statement
CONFLICT OF INTEREST The authors have no conflicts of interest to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

