Improving the Feasibility of Glaucoma Clinical Trials Using Trend-Based Visual Field Progression Endpoints
- PMID: 32632403
- PMCID: PMC7337274
- DOI: 10.1016/j.ogla.2019.01.004
Improving the Feasibility of Glaucoma Clinical Trials Using Trend-Based Visual Field Progression Endpoints
Abstract
Purpose: There have been concerns that short-term clinical trials for evaluating new treatments in glaucoma would require prohibitively large sample sizes when using visual field endpoints, given that glaucoma is often a slowly progressive disease. This study sought to determine the required sample size for such trials using event-based analyses, and whether it can be reduced using trend-based analyses.
Design: Longitudinal, observational study.
Participants: 321 eyes of 240 glaucoma participants followed under routine clinical care using 242 visual field for an average of 10 years.
Methods: Sample size requirements were derived using computer simulations that reconstructed "real-world" visual fields by combining estimates of point-wise variability according to different threshold levels and rates of change obtained from the clinical glaucoma cohort. A clinical trial lasting 2 years with testing every 3 months was simulated, assuming that the new treatment halted visual field change in various percentages of participants (or "responders"). Treatment efficacy was evaluated by: (a) Difference in incidence of point-wise event-based progression (similar to the commercially available Guided Progression Analysis), and (b) Difference in rate of visual field mean deviation (MD) change between groups using linear mixed models (LMMs).
Main outcome measures: Sample size to detect a statistically significance difference between groups.
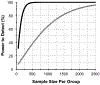
Results: Between-group trend-based analyses using LMMs reduced sample size requirements by 85-90% across the range of new treatment effects when compared to the conventional point-wise event-based analysis. To detect the effect of a new treatment that halted progression in 30% of the participants under routine clinical care (equal to a 30% reduction in average rate of MD change) with 90% power, for example, 1924 participants would be required per group using event-based analysis, but only 277 participants per group if LMMs were used.
Conclusions: The feasibility of future glaucoma clinical trials can be substantially improved by evaluating differences in the rate of visual field change between groups.
Keywords: Visual fields; clinical trials; glaucoma; sample size.
Figures
Similar articles
-
Sample Size Requirements of Glaucoma Clinical Trials When Using Combined Optical Coherence Tomography and Visual Field Endpoints.Sci Rep. 2019 Dec 11;9(1):18886. doi: 10.1038/s41598-019-55345-x. Sci Rep. 2019. PMID: 31827169 Free PMC article.
-
Comparing 10-2 and 24-2 Visual Fields for Detecting Progressive Central Visual Loss in Glaucoma Eyes with Early Central Abnormalities.Ophthalmol Glaucoma. 2019 Mar-Apr;2(2):95-102. doi: 10.1016/j.ogla.2019.01.003. Epub 2019 Jan 14. Ophthalmol Glaucoma. 2019. PMID: 31742250 Free PMC article.
-
Frequency of Testing to Detect Visual Field Progression Derived Using a Longitudinal Cohort of Glaucoma Patients.Ophthalmology. 2017 Jun;124(6):786-792. doi: 10.1016/j.ophtha.2017.01.027. Epub 2017 Mar 6. Ophthalmology. 2017. PMID: 28268099
-
[Aiming for zero blindness].Nippon Ganka Gakkai Zasshi. 2015 Mar;119(3):168-93; discussion 194. Nippon Ganka Gakkai Zasshi. 2015. PMID: 25854109 Review. Japanese.
-
[Visual field progression in glaucoma: cluster analysis].J Fr Ophtalmol. 2012 Nov;35(9):735-41. doi: 10.1016/j.jfo.2011.10.011. Epub 2012 Jul 6. J Fr Ophtalmol. 2012. PMID: 22771181 Review. French.
Cited by
-
UWHVF: A Real-World, Open Source Dataset of Perimetry Tests From the Humphrey Field Analyzer at the University of Washington.Transl Vis Sci Technol. 2022 Jan 3;11(1):2. doi: 10.1167/tvst.11.1.1. Transl Vis Sci Technol. 2022. PMID: 34978561 Free PMC article.
-
Short-term Assessment of Glaucoma Progression in Clinical Trials Using Trend-based Visual Field Progression Analysis.Ophthalmol Sci. 2024 Nov 19;5(2):100656. doi: 10.1016/j.xops.2024.100656. eCollection 2025 Mar-Apr. Ophthalmol Sci. 2024. PMID: 39886357 Free PMC article.
-
Validation of Rates of Mean Deviation Change as Clinically Relevant End Points for Glaucoma Progression.Ophthalmology. 2023 May;130(5):469-477. doi: 10.1016/j.ophtha.2022.12.025. Epub 2022 Dec 24. Ophthalmology. 2023. PMID: 36574847 Free PMC article.
-
Loss of OCT Outer Retinal Bands as Potential Clinical Trial Endpoints in Intermediate Age-Related Macular Degeneration.Ophthalmol Sci. 2025 Mar 17;5(4):100769. doi: 10.1016/j.xops.2025.100769. eCollection 2025 Jul-Aug. Ophthalmol Sci. 2025. PMID: 40308329 Free PMC article.
-
Cholinergic nervous system and glaucoma: From basic science to clinical applications.Prog Retin Eye Res. 2019 Sep;72:100767. doi: 10.1016/j.preteyeres.2019.06.003. Epub 2019 Jun 23. Prog Retin Eye Res. 2019. PMID: 31242454 Free PMC article. Review.
References
-
- Weinreb RN, Liebmann JM, Cioffi GA, et al. Oral Memantine for the Treatment of Glaucoma: Design and Results of 2 Randomized, Placebo-Controlled, Phase 3 Studies. Ophthalmology 2018;125(12):1874–85. - PubMed
-
- Garway-Heath D, Crabb D, Bunce C, et al. Latanoprost treatment for open angle glaucoma. The United Kingdom Glaucoma Treatment Study: a multicentre, randomised, placebo-controlled clinical trial. Lancet 2015;385(9975):1295–304. - PubMed
-
- Quigley HA. Clinical trials for glaucoma neuroprotection are not impossible. Curr Opin Ophthalmol 2012;23(2):144–54. - PubMed
-
- Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol 1999;117(5):573–83. - PubMed
-
- Leske MC, Heijl A, Hyman L, et al. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology 1999;106(11):2144–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical