Intranasal Dexamethasone Reduces Mortality and Brain Damage in a Mouse Experimental Ischemic Stroke Model
- PMID: 32632775
- PMCID: PMC7851226
- DOI: 10.1007/s13311-020-00884-9
Intranasal Dexamethasone Reduces Mortality and Brain Damage in a Mouse Experimental Ischemic Stroke Model
Abstract
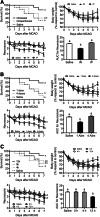
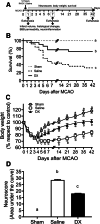
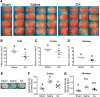
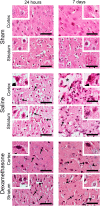
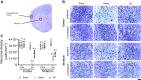
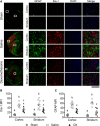
Neuroinflammation triggered by the expression of damaged-associated molecular patterns released from dying cells plays a critical role in the pathogenesis of ischemic stroke. However, the benefits from the control of neuroinflammation in the clinical outcome have not been established. In this study, the effectiveness of intranasal, a highly efficient route to reach the central nervous system, and intraperitoneal dexamethasone administration in the treatment of neuroinflammation was evaluated in a 60-min middle cerebral artery occlusion (MCAO) model in C57BL/6 male mice. We performed a side-by-side comparison using intranasal versus intraperitoneal dexamethasone, a timecourse including immediate (0 h) or 4 or 12 h poststroke intranasal administration, as well as 4 intranasal doses of dexamethasone beginning 12 h after the MCAO versus a single dose at 12 h to identify the most effective conditions to treat neuroinflammation in MCAO mice. The best results were obtained 12 h after MCAO and when mice received a single dose of dexamethasone (0.25 mg/kg) intranasally. This treatment significantly reduced mortality, neurological deficits, infarct volume size, blood-brain barrier permeability in the somatosensory cortex, inflammatory cell infiltration, and glial activation. Our results demonstrate that a single low dose of intranasal dexamethasone has neuroprotective therapeutic effects in the MCAO model, showing a better clinical outcome than the intraperitoneal administration. Based on these results, we propose a new therapeutic approach for the treatment of the damage process that accompanies ischemic stroke.
Keywords: Dexamethasone; MCAO; inflammation; intranasal administration; ischemic stroke.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Institute for Health Metrics and Evaluation (IHME) Findings from the Global Burden of Disease Study 2017. Seattle, WA: IHME; 2018.
-
- López-Espuela F, Pedrera-Zamorano JD, Jiménez-Caballero PE, et al. Functional status and disability in patients after acute stroke: a longitudinal study. Am J Crit Care. 2016;25:144–151. - PubMed
-
- Noe-Sebastian E, Balasch-Bernat M, Colomer-Font C, et al. Disability after stroke: a longitudinal study in moderate and severe stroke patients included in a multidisciplinary rehabilitation program. Rev Neurol. 2017;64:385–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

