A real-world analysis of patient-reported outcomes in patients with migraine by preventive treatment eligibility status in the US and Europe
- PMID: 32632891
- PMCID: PMC7338330
- DOI: 10.1186/s41687-020-00221-w
A real-world analysis of patient-reported outcomes in patients with migraine by preventive treatment eligibility status in the US and Europe
Abstract
Background: Migraine has a severe impact on health-related quality of life (HRQoL) affecting physical, emotional, and social aspects of daily living of an individual. Preventive treatment has been demonstrated to improve HRQoL by reducing the frequency of migraine headache days.
Methods: The study used data from 2017 Adelphi Migraine Disease Specific Program, which is a cross-sectional survey of physicians and their consulting patients with migraine in the United States (US) and five European countries (EU [Germany, France, UK, Italy and Spain]). Objectives were to evaluate patient-reported outcome (PRO) measures in the following two subgroups and by region (US and EU): (i) patients who are eligible for migraine preventive treatment (≥4 migraine headache days/month), and (ii) patients who are non-eligible for preventive treatment (< 4 migraine headache days/month). Patient-reported outcome measures that were assessed included the following: Migraine-Specific Quality-of-Life Questionnaire Version 2.1, Migraine Disability Assessment Scale (MIDAS), European Quality of Life-5 Dimensions-5 Levels version, and Work Productivity and Activity Impairment.
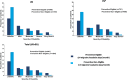
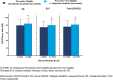
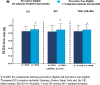
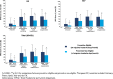
Results: In total, 5462 patients (US = 1373; EU = 4089) were included in the study (preventive eligible: US = 584; EU = 1942; preventive non-eligible: US = 789; EU = 2147). In the US and EU, preventive eligible patients were significantly more likely to have worse disability as measured by MIDAS than non-eligible patients; preventive eligible patients also had significantly greater functional impairment, worse health utility, and overall greater work impairment (p < 0.0001). Among patients who were preventive eligible, a larger proportion of patients in the US reported that migraine forced them to reduce the number of hours worked as compared with the EU population (29.0% vs 24.7%).
Conclusion: Patients who were preventive eligible (≥4 migraine headache days/month) demonstrated greater burden of disease across multiple PRO measures; trends were similar across the US and the five EU countries.
Keywords: Burden of disease; Migraine; Patient-reported outcomes; Preventive eligibility.
Conflict of interest statement
This work was supported by Eli Lilly and Company, Indianapolis, Indiana, USA and Adelphi Mill, Bollington, UK. Ford JH, Foster SA, Nichols RM, Tockhorn-Heidenreich A, Wenyu Ye are full-time employees of Eli Lilly and Company and/or one of its subsidiaries and may hold company stocks. Cotton S and Jackson J are employees of Adelphi Real World who received funding from Eli Lilly for this study.
Figures

References
LinkOut - more resources
Full Text Sources

