Estrogen receptor alpha, G-protein coupled estrogen receptor 1, and aromatase: Developmental, sex, and region-specific differences across the rat caudate-putamen, nucleus accumbens core and shell
- PMID: 32632943
- PMCID: PMC7775873
- DOI: 10.1002/cne.24978
Estrogen receptor alpha, G-protein coupled estrogen receptor 1, and aromatase: Developmental, sex, and region-specific differences across the rat caudate-putamen, nucleus accumbens core and shell
Abstract
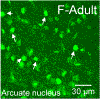
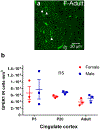
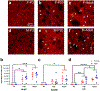
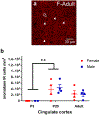
Sex steroid hormones such as 17β-estradiol (estradiol) regulate neuronal function by binding to estrogen receptors (ERs), including ERα and GPER1, and through differential production via the enzyme aromatase. ERs and aromatase are expressed across the nervous system, including in the striatal brain regions. These regions, comprising the nucleus accumbens core, shell, and caudate-putamen, are instrumental for a wide-range of functions and disorders that show sex differences in phenotype and/or incidence. Sex-specific estrogen action is an integral component for generating these sex differences. A distinctive feature of the striatal regions is that in adulthood neurons exclusively express membrane but not nuclear ERs. This long-standing finding dominates models of estrogen action in striatal regions. However, the developmental etiology of ER and aromatase cellular expression in female and male striatum is unknown. This omission in knowledge is important to address, as developmental stage influences cellular estrogenic mechanisms. Thus, ERα, GPER1, and aromatase cellular immunoreactivity was assessed in perinatal, prepubertal, and adult female and male rats. We tested the hypothesis that ERα, GPER1, and aromatase exhibits sex, region, and age-specific differences, including nuclear expression. ERα exhibits nuclear expression in all three striatal regions before adulthood and disappears in a region- and sex-specific time-course. Cellular GPER1 expression decreases during development in a region- but not sex-specific time-course, resulting in extranuclear expression by adulthood. Somatic aromatase expression presents at prepuberty and increases by adulthood in a region- but not sex-specific time-course. These data indicate that developmental period exerts critical sex-specific influences on striatal cellular estrogenic mechanisms.
Keywords: RRID AB_1141090; RRID AB_310305; RRID AB_566942; aromatase; estrogen receptor; rat; sex differences; striatum.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Figures


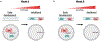
References
-
- Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, … Lenfant F (2014). Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A, 111(2), E283–290. doi:10.1073/pnas.1322057111 - DOI - PMC - PubMed
-
- Almey A, Cannell E, Bertram K, Filardo E, Milner TA, & Brake WG (2014). Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology, 155(11), 4422–4432. doi:10.1210/en.2014-1463 - DOI - PMC - PubMed
-
- Almey A, Filardo EJ, Milner TA, & Brake WG (2012). Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology, 153(11), 5373–5383. doi:10.1210/en.2012-1458 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

