Interventions for mycosis fungoides
- PMID: 32632956
- PMCID: PMC7389258
- DOI: 10.1002/14651858.CD008946.pub3
Interventions for mycosis fungoides
Abstract
Background: Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma, a malignant, chronic disease initially affecting the skin. Several therapies are available, which may induce clinical remission for a time. This is an update of a Cochrane Review first published in 2012: we wanted to assess new trials, some of which investigated new interventions.
Objectives: To assess the effects of interventions for MF in all stages of the disease.
Search methods: We updated our searches of the following databases to May 2019: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We searched 2 trials registries for additional references. For adverse event outcomes, we undertook separate searches in MEDLINE in April, July and November 2017.
Selection criteria: Randomised controlled trials (RCTs) of local or systemic interventions for MF in adults with any stage of the disease compared with either another local or systemic intervention or with placebo.
Data collection and analysis: We used standard methodological procedures expected by Cochrane. The primary outcomes were improvement in health-related quality of life as defined by participants, and common adverse effects of the treatments. Key secondary outcomes were complete response (CR), defined as complete disappearance of all clinical evidence of disease, and objective response rate (ORR), defined as proportion of patients with a partial or complete response. We used GRADE to assess the certainty of evidence and considered comparisons of psoralen plus ultraviolet A (PUVA) light treatment as most important because this is first-line treatment for MF in most guidelines.
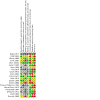
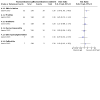
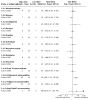
Main results: This review includes 20 RCTs (1369 participants) covering a wide range of interventions. The following were assessed as either treatments or comparators: imiquimod, peldesine, hypericin, mechlorethamine, nitrogen mustard and intralesional injections of interferon-α (IFN-α) (topical applications); PUVA, extracorporeal photopheresis (ECP: photochemotherapy), and visible light (light applications); acitretin, bexarotene, lenalidomide, methotrexate and vorinostat (oral agents); brentuximab vedotin; denileukin diftitox; mogamulizumab; chemotherapy with cyclophosphamide, doxorubicin, etoposide, and vincristine; a combination of chemotherapy with electron beam radiation; subcutaneous injection of IFN-α; and intramuscular injections of active transfer factor (parenteral systemics). Thirteen trials used an active comparator, five were placebo-controlled, and two compared an active operator to observation only. In 14 trials, participants had MF in clinical stages IA to IIB. All participants were treated in secondary and tertiary care settings, mainly in Europe, North America or Australia. Trials recruited both men and women, with more male participants overall. Trial duration varied from four weeks to 12 months, with one longer-term study lasting more than six years. We judged 16 trials as at high risk of bias in at least one domain, most commonly performance bias (blinding of participants and investigators), attrition bias and reporting bias. None of our key comparisons measured quality of life, and the two studies that did presented no usable data. Eighteen studies reported common adverse effects of the treatments. Adverse effects ranged from mild symptoms to lethal complications depending upon the treatment type. More aggressive treatments like systemic chemotherapy generally resulted in more severe adverse effects. In the included studies, CR rates ranged from 0% to 83% (median 31%), and ORR ranged from 0% to 88% (median 47%). Five trials assessed PUVA treatment, alone or combined, summarised below. There may be little to no difference between intralesional IFN-α and PUVA compared with PUVA alone for 24 to 52 weeks in CR (risk ratio (RR) 1.07, 95% confidence interval (CI) 0.87 to 1.31; 2 trials; 122 participants; low-certainty evidence). Common adverse events and ORR were not measured. One small cross-over trial found once-monthly ECP for six months may be less effective than twice-weekly PUVA for three months, reporting CR in two of eight participants and ORR in six of eight participants after PUVA, compared with no CR or ORR after ECP (very low-certainty evidence). Some participants reported mild nausea after PUVA but no numerical data were given. One participant in the ECP group withdrew due to hypotension. However, we are unsure of the results due to very low-certainty evidence. One trial comparing bexarotene plus PUVA versus PUVA alone for up to 16 weeks reported one case of photosensitivity in the bexarotene plus PUVA group compared to none in the PUVA-alone group (87 participants; low-certainty evidence). There may be little to no difference between bexarotene plus PUVA and PUVA alone in CR (RR 1.41, 95% CI 0.71 to 2.80) and ORR (RR 0.94, 95% CI 0.61 to 1.44) (93 participants; low-certainty evidence). One trial comparing subcutaneous IFN-α injections combined with either acitretin or PUVA for up to 48 weeks or until CR indicated there may be little to no difference in the common IFN-α adverse effect of flu-like symptoms (RR 1.32, 95% CI 0.92 to 1.88; 82 participants). There may be lower CR with IFN-α and acitretin compared with IFN-α and PUVA (RR 0.54, 95% CI 0.35 to 0.84; 82 participants) (both outcomes: low-certainty evidence). This trial did not measure ORR. One trial comparing PUVA maintenance treatment to no maintenance treatment, in participants who had already had CR, did report common adverse effects. However, the distribution was not evaluable. CR and OR were not assessable. The range of treatment options meant that rare adverse effects consequently occurred in a variety of organs.
Authors' conclusions: There is a lack of high-certainty evidence to support decision making in the treatment of MF. Because of substantial heterogeneity in design, missing data, small sample sizes, and low methodological quality, the comparative safety and efficacy of these interventions cannot be reliably established on the basis of the included RCTs. PUVA is commonly recommended as first-line treatment for MF, and we did not find evidence to challenge this recommendation. There was an absence of evidence to support the use of intralesional IFN-α or bexarotene in people receiving PUVA and an absence of evidence to support the use of acitretin or ECP for treating MF. Future trials should compare the safety and efficacy of treatments to PUVA, as the current standard of care, and should measure quality of life and common adverse effects.
Trial registration: ClinicalTrials.gov NCT00030589 NCT00050999 NCT01578499 NCT00630903 NCT00091208 NCT01386398 NCT01625455 NCT01738594 NCT02213861 NCT02301494 NCT02323659 NCT02448381 NCT02811783 NCT02943642 NCT02953301 NCT03011814 NCT03292406 NCT03454945 NCT03713320.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Arash Valipour: I received travel expenses and accommodation for the annual German Dermatology Society Meeting 2019 from UCB Pharma, but this company is not involved in the development of drugs for mycosis fungoides. I attended a meeting called 'Inflammation campus' (a dermatology/rheumatology‐oriented medical meeting) in July 2015, which was organised by Pfizer. Pfizer paid for my travel expenses and accommodation. Manuel Jäger: nothing to declare. Peggy Wu: nothing to declare. Jochen Schmitt: I acted as a consultant for Lilly and Sanofi and received institutional grants for investigator‐initiated trials outside the submitted work from Novartis, Sanofi, ALK, and Pfizer. Charles Bunch: nothing to declare. Tobias Weberschock: nothing to declare.
Clinical referee, Prof Sean Whittaker: "I led and co‐authored the updated U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas, which were published in 2018 (Gilson 2019)."
Figures





































Update of
-
Interventions for mycosis fungoides.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008946. doi: 10.1002/14651858.CD008946.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2020 Jul 7;7:CD008946. doi: 10.1002/14651858.CD008946.pub3. PMID: 22972128 Updated.
References
References to studies included in this review
Bagot 2017 {published and unpublished data}
-
- Bagot M, Hasan B, Whittaker S, Beylot-Barry M, Knobler R, Shah E, et al. A phase III study of lenalidomide maintenance after debulking therapy in patients with advanced cutaneous T-cell lymphoma - EORTC 21081 (NCT01098656): results and lessons learned for future trial designs. European Journal of Dermatology 2017;27(3):286–94. [CENTRAL: CN-01395486] [PMID: ] - PubMed
-
- NCT01098656. A phase III study of lenalidomide maintenance after debulking therapy in patients with advanced cutaneous T-Cell lymphoma. clinicaltrials.gov/ct2/show/NCT01098656 (first received 5 April 2010). [NCT01098656]
Child 2004 {published data only (unpublished sought but not used)}
-
- Child FJ, Mitchell TJ, Whittaker SJ, Scarisbrick JJ, Seed PT, Russel-Jones R. A randomized cross-over study to compare PUVA and extracorporeal photopheresis in the treatment of plaque stage (T2) mycosis fungoides. Clinical & Experimental Dermatology 2004;29(3):231-6. [CENTRAL: CN-00468455] [PMID: ] - PubMed
-
- Child FJ, Mitchell TJ, Whittaker SJ, Watkins P, Seed P, Russell-Jones R. A randomised cross-over study to compare Puva and extracorporeal photopheresis (ECP) in the treatment of plaque stage (T2) mycosis fungoides. British Journal of Dermatology 2001;145(Suppl 59):16. [CENTRAL: CN-00430854] - PubMed
Chong 2004 {published data only (unpublished sought but not used)}
-
- Chong A, Loo WJ, Banney L, Grant JW, Norris PG. Imiquimod 5% cream in the treatment of mycosis fungoides - a pilot study. Journal of Dermatological Treatment 2004;15(2):118-9. [CENTRAL: CN-00467258] [PMID: ] - PubMed
Duvic 2001 {published data only}
-
- Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, et al. Phase 2 and 3 clinical trial of oral bexarotene (Targretin capsules) for the treatment of refractory or persistent early-stage cutaneous T-cell lymphoma. Archives of Dermatology 2001;137(5):581-93. [CENTRAL: CN-00347816] [PMID: ] - PubMed
-
- Prince HM, McCormack C, Ryan G, Baker C, Rotstein H, Davison J, et al. Bexarotene capsules and gel for previously treated patients with cutaneous T-cell lymphoma: results of the Australian patients treated on phase II trials. Australasian Journal of Dermatology 2001;42(2):91-7. [CENTRAL: CN-00442169] [PMID: ] - PubMed
Duvic 2001a {published data only}
-
- Duvic M, Olsen EA, Omura GA, Maize JC, Vonderheid EC, et al. A phase III, randomized, double-blind, placebo-controlled study of peldesine (BCX-34) cream as topical therapy for cutaneous T-cell lymphoma. Journal of the American Academy of Dermatology 2001;44(6):940-7. [CENTRAL: CN-00348073] [PMID: ] - PubMed
Guitart 2002 {published and unpublished data}
-
- Guitart J, Tucker R, Stevens V. Low dose bexarotene (Targretin®) capsules and phototherapy for early stage cutaneous T-Cell lymphoma. Journal of Investigative Dermatology 2002;119(1):241. [CENTRAL: CN-00794450]
-
- NCT00030589. A muliticenter, dose-randomized evaluation of Targretin capsules plus PUVA in patients with stage IB - IIA cutaneous T-Cell lymphoma. clinicaltrials.gov/ct/show/NCT00030589 (first received 27 Jaunry 2003).
Kaye 1989 {published data only}
-
- Kaye F, Eddy J, Ihde DC, Steinberg S, Fischmann AB, Glatstein E, et al. Conservative vs. aggressive therapy in mycosis fungoides. Proceedings of the American Society of Clinical Oncology 1989;8:257. [CENTRAL: CN-00694960]
-
- Kaye F, Ihde D, Fischmann A, Glatstein E, Minna J, Schechter G, et al. A randomized trial comparing conservative and aggressive therapy in mycosis fungoides (MF). Proceedings of the American Society of Clinical Oncology 1986;5:195. [CENTRAL: CN-00695166]
-
- Kaye FJ, Bunn PA, Steinberg SM, Stocker JL, Ihde DC, Fischmann AB, et al. A randomized trial comparing combination electron-beam radiation and chemotherapy with topical therapy in the initial treatment of mycosis fungoides. New England Journal of Medicine 1989;321(26):1784-90. [CENTRAL: CN-00064211] [PMID: ] - PubMed
Kim 2018 {published data only}
-
- Bagot M, Dalle S, Sokol L, Tsianakas A, Musiek A, Ortiz-Romero P, et al. Long-term clinical benefit to anti-CCR4 mogamulizumab: results from the phase 3 mavoric study in previously treated cutaneous t-cell lymphoma (CTCL). Blood 2018;132(Supp 1):2901. [DOI: 10.1182/blood-2018-99-118473] - DOI
-
- Cowan R, Scarisbrick J, Dwyer K, Leoni M, Grebennik D, Morris S. Mogamulizumab demonstrates significant improvement in PFS compared to vorinostat in patients with previously treated cutaneous T-cell lymphoma (CTCL). British Journal of Haematology 2018;181(S1):77–8. [DOI: 10.1111/bjh.15226] - DOI
-
- Kim YH, Bagot M, Pinter-Brown L, Rook AH, Porcu P, Horwitz SM, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncology 2018;19(9):1192-1204. [PMID: ] - PubMed
-
- NCT01728805. Study of KW-0761 versus vorinostat in relapsed/refractory CTCL. clinicaltrials.gov/ct2/show/NCT01728805 (first received 20 November 2012).
-
- Porcu P, Hudgens S, Quaglino P, Cowan R, Floden L, Leoni M, et al. Quality of life in cutaneous T-cell lymphoma subjects treated with anti-CCR4 monoclonal antibody mogamulizumab versus vorinostat: results from the phase 3 MAVORIC trial. Journal of Clinical Oncology 2018;36(15):-. [DOI: 10.1097/HS9.0000000000000060] - DOI
Lessin 2013 {published data only}
-
- Lessin S, Duvic M, Guitart J, Pandya A, Strober B, Olsen E, et al. Positive results of a pivotal phase ii trial testing a manufactured 0.02% Mechlorethamine gel in mycosis fungoides (mf). Journal of Investigative Dermatology 2011;131(Suppl 1):S84. [CENTRAL: CN-00843792]
-
- Lessin SR, Duvic M, Guitart J, Pandya AG, Strober BE, Olsen EA, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatology 2013;149(1):25-32. [CENTRAL: CN-00859487] [PMID: ] - PMC - PubMed
-
- NCT00168064. Safety and efficacy of nitrogen mustard in treatment of mycosis fungoides. clinicaltrials.gov/ct2/show/NCT00168064 (first received 14 September 2005).
Olsen 2001 {published and unpublished data}
-
- Duvic M, Kuzel TM, Olsen EA, Martin AG, Foss FM, Kim YH, et al. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK). Clinical Lymphoma 2002;2(4):222-8. [CENTRAL: CN-00514479] [PMID: ] - PubMed
-
- Kuzel T, Olsen E, Martin A, Kim Y, Duvic M, Frankel A, et al. Pivotal phase III trial of two dose levels of DAB389IL-2 (Ontak®) for the treatment of mycosis fungoides (MF). Blood 1997;90(10 Suppl 1 (Pt 1)):586a. [CENTRAL: CN-00518069]
-
- NTC00050999. Study of ONTAK (Denileukin Diftitox) in cutaneous T-Cell lymphoma (CTCL) patients. clinicaltrials.gov/ct2/show/NCT00050999 (first received 3 January 2003).
-
- Olsen E, Duvic M, Frankel A, Kim Y, Martin A, Vonderheid E, et al. Pivotal phase III trial of two dose levels of denileukin diftitox for the treatment of cutaneous T-cell lymphoma. Journal of Clinical Oncology 2001;19(2):376-88. [CENTRAL: CN-00328506] [PMID: ] - PubMed
-
- Olsen E, Duvic M, Martin A. Pivotal phase III trial of two dose levels of DAB 389 IL-2 (ONTAK) for the treatment of cutaneous T-cell lymphoma (CTCL). Journal of Investigative Dermatology 1998;110(4):678. [CENTRAL: CN-00353452]
Prince 2017 {published data only}
-
- Horwitz S, Whittaker S, Duvic M, Dummer R, Kim YH, Scarisbrick J, et al. Response by stage in CD30-positive (CD30+) cutaneous T cell lymphoma (CTCL) patients receiving brentuximab vedotin (BV) vs physician's choice (PC) in the phase 3 ALCANZA study. In: Hematological oncology. Conference: 14th international conference on malignant lymphoma palazzo dei congressi. Switzerland. Vol. 35. 2017:245–7. [CENTRAL: CN-01408605] [DOI: 10.1002/hon.2438] - DOI
-
- Horwitz SM, Akilov OE, Fisher D, Kuzel TM, Wang Y, Palanca-Wessels MC, et al. Brentuximab vedotin or physician’s choice in CD30-positivecutaneous T-cell lymphoma (ALCANZA): an international,open-label, randomised, phase 3, multicentre trial. Lancet 2017;390(10094):555-66. [CENTRAL: CN-01403731] - PubMed
-
- Horwitz SM, Scarisbrick JJ, Dummer R, Duvic M, Kim YH, Walewski J, et al. Updated analyses of the international, open-label, randomized, phase 3 alcanza study: longer-term evidence for superiority of brentuximab vedotin versus methotrexate or bexarotene for CD30-positive cutaneous T-cell lymphoma (CTCL). In: Blood. Conference: 59th annual meeting of the american society of hematology, ASH 2017. United states. Vol. 130. 2017. [CENTRAL: CN-01450312]
-
- Kim YH, Prince HM, Whittaker S, Horwitz SM, Duvic M, Scarisbrick J, et al. Brentuximab vedotin vs physician's choice in CTCL patients from the phase 3 ALCANZA study: analysis of outcomes by CD30 expression. In: Hematological Oncology. Conference: 14th international conference on malignant lymphoma palazzo dei congressi. Switzerland. Vol. 35. 2017:77–8. [CENTRAL: CN-01408482] [DOI: 10.1002/hon.2437] - DOI
-
- Kim YH, Prince HM, Whittaker S, Horwitz SM, Duvic M, Scarisbrick J, et al. Outcomes by CD30 expression in patients with CTCL receiving brentuximab vedotin (BV) vs physician's choice (PC) in the Phase 3 ALCANZA study. Journal of Clinical Oncology 2017;35(15 Supplement 1):7517. [EMBASE: 617388795]
Rook 2010 {published and unpublished data}
-
- Rook AH, Wood GS, Duvic M, Vonderheid EC, Tobia A, Cabana B. A phase II placebo-controlled study of photodynamic therapy with topical hypericin and visible light irradiation in the treatment of cutaneous T-cell lymphoma and psoriasis. Journal of the American Academy of Dermatology 2010;63(6):984-90. [CENTRAL: CN-00772559] [PMID: ] - PubMed
Stadler 1998 {published and unpublished data}
-
- Otte HG, Kuhl S, Stadler R, Luger T, Henz B, Sterry W. Treatment of cutaneous T-cell lymphoma with Interferon alpha and PUVA versus Interferon alpha and Acitretin - results of a prospective randomised multi-centre study [Behandlung des kutanen T-Zell-Lymphoms mit Interferon alpha und PUVA versus Interferon alpha und Acirentin - Ergebnisse einer prospektiv-randomisierten Multicenter-Studie]. Der Hautarzt 1997;48(Suppl):S97. [CENTRAL: CN-00495120]
-
- Otte HG, Stadier R, Luger T, Henz B, Sterry W. Open controlled clinical trial on the use of interferon plus acitretin versus interferon plus PUVA in patients with cutaneous T-cell lymphoma (CTCL). Annals of Oncology 1996;7(Suppl 3):66. [CENTRAL: CN-00308243] - PubMed
-
- Stadler R, Otte HG, Luger T, Henz BM, Kuhl P, Zwingers T, et al. Prospective randomized multicenter clinical trial on the use of interferon -2a plus acitretin versus interferon -2a plus PUVA in patients with cutaneous T-cell lymphoma stages I and II. Blood 1998;92(10):3578-81. [CENTRAL: CN-00156682] [PMID: ] - PubMed
Stadler 2006 {published and unpublished data}
-
- Bohmeyer J, Otte HG, Stadler R, Luger T, Sterry W. Therapy of cutaneous T-cell lymphoma with PUVA versus interferon alfa plus PUVA: first results. Journal of the European Academy of Dermatology & Venereology 1999;12(Suppl 2):S268. [CENTRAL: CN-00478472]
-
- Bohmeyer J, Stadler R, Luger T, Sterry W. Prospective randomized multicentre therapy optimizing protocol for therapy of cutaneous t-cell-lymphoma with PUVA versus Interferon α + PUVA [Prospectiv randomisiertes, multizentrisches Therapieoptimierungsprotokoll zur Therapie kutaner T-Zell-Lymphome mit PUVA versus Interferon α + PUVA]. Zeitschrift für Hautkrankheiten 2001;76(Suppl 1):S55. [CENTRAL: CN-01446038]
-
- Kremer A, Bohmeyer J, Stadler R, Luger T, Sterry W. Prospective randomized multicentre therapy optimizing protocol for therapy of cutaneous t-cell-lymphoma with PUVA versus Interferon α + PUVA [Prospectiv randomisiertes, multizentrisches Therapieoptimierungsprotokoll zur Therapie kutaner T-Zell-Lymphome mit PUVA versus Interferon α + PUVA]. Journal der Deutschen Dermatologischen Gesellschaft 2003;1(Suppl 1):S99. [CENTRAL: CN-01446037]
-
- Otte HG, Stadler R, Luger T, Sterry W. Therapy of cutaneous t-cell-lymphoma with PUVA versus Interferon α plus PUVA: first results of a prospective randomized multicentre therapy optimizing protocol [Therapie kutaner T-Zell-Lymphome mit PUVA versus Interferon α plus PUVA: Erste Ergebnisse des prospektiven randomisierten multizentrischen Therapieoptimierungsprotokolls]. Der Hautarzt 1999;50(Suppl 1):S2. [CENTRAL: CN-01457423]
-
- Otte HG, Stadler R. Combination therapy of cutaneous t-cell-lymphoma with interferon alfa 2a and PUVA [Kombinationstherapie kutaner T-Zell-Lymphome mit Interferon Alfa 2a und PUVA]. Zentralblatt Haut- und Geschlechtskrankheiten 1993;162(Suppl):P2.03.08. [CENTRAL: CN-01457422]
Thestrup‐Pedersen 1982 {published and unpublished data}
-
- Thestrup-Pedersen K, Grunnet E, Zachariae H. Transfer factor therapy in mycosis fungoides: a double-blind study. Acta Dermato-Venereologica 1982;62(1):47-53. [CENTRAL: CN-00027377] [PMID: ] - PubMed
Vieyra‐Garcia 2019 {published data only}
-
- NCT01686594. A multi-center, randomized study on oral 8-methoxypsoralen plus UVA with or without maintenance therapy in mycosis fungoides EORTC/ISCL stage IA to IIB. clinicaltrials.gov/ct2/show/NCT01686594 (first received 18 September 2012).
-
- Vieyra-Garcia P, Fink-Puches R, Porkert S, Lang R, Pöchlauer S, Ratzinger G, et al. Evaluation of low-dose, low-frequency oral psoralen-UV-A treatment with or without maintenance on early-stage mycosis fungoides: A randomized clinical trial. JAMA Dermatology 2019;155(5):538-47. [PMID: ] - PMC - PubMed
Vonderheid 1987 {published data only (unpublished sought but not used)}
-
- Vonderheid EC, Thompson R, Smiles KA, Lattanand A. Recombinant interferon alfa-2b in plaque-phase mycosis fungoides. Intralesional and low-dose intramuscular therapy. Archives of Dermatology 1987;123(6):757-63. [CENTRAL: CN-00047990] [PMID: ] - PubMed
Whittaker 2012 {published data only}
-
- NCT00056056. Ultraviolet light therapy using methoxsalen with or without Bexarotene in treating patients with mycosis fungoides. clinicaltrials.gov/ct2/show/NCT00056056 (first received 7 March 2003).
-
- Whittaker S, Ortiz P, Dummer R, Ranki A, Hasan B, Meulemans B, et al. Efficacy and safety of bexarotene combined with psoralen-ultraviolet A (PUVA) compared with PUVA treatment alone in stage IB-IIA mycosis fungoides: final results from the EORTC Cutaneous Lymphoma Task Force phase III randomized clinical trial 21011 (NCT00056056). British Journal of Dermatology 2012;167(3):678-87. [CENTRAL: CN-00856432] [PMID: ] - PubMed
-
- Whittaker S, Ortiz-Romero PL, Dummer R, Ranki A, Hasan B, Meulemans B, et al. Efficacy and safety of bexarotene combined with psoralen/ultraviolet A light (PUVA) compared to PUVA treatment alone in stage IB-IIa mycosis fungoides (MF): Final results from EORTC cutaneous lymphoma task force (CLTF) phase III clinical trial 21011. Journal of Clinical Oncology 2012;30(15 Suppl 1):8076. - PubMed
Wolff 1985 {published data only (unpublished sought but not used)}
-
- Wolff JM, Zitelli JA, Rabin BS, Smiles KA, Abell E. Intralesional interferon in the treatment of early mycosis fungoides. Journal of the American Academy of Dermatology 1985;13(4):604-12. [CENTRAL: CN-00040943] [PMID: ] - PubMed
Wozniak 2008 {published data only (unpublished sought but not used)}
-
- Wozniak MB, Tracey L, Ortiz-Romero PL, Montes S, Alvarez M, Fraga J, et al. Psoralen plus ultraviolet A +/- interferon-alpha treatment resistance in mycosis fungoides: the role of tumour microenvironment, nuclear transcription factor-kappaB and T-cell receptor pathways. British Journal of Dermatology 2008;160(1):92-102. [CENTRAL: CN-00668828] [PMID: ] - PubMed
References to studies excluded from this review
Anonymous 1982 {published data only}
-
- No authors listed. Comparison of the use of teniposide and vincristine in combination chemotherapy for non-Hodgkin's lymphoma. Cancer Treatment Reports 1982;66(1):49-55. - PubMed
Anonymous 2000 {published data only}
-
- Anonymous. Bexarotene (Targretin) for cutaneous T-cell lymphoma. Medical Letter on Drugs and Therapeutics 2000;42(1075):31-2. [PMID: ] - PubMed
Argyropoulos 1979 {published data only}
-
- Argyropoulos CL, Lamberg SI, Clendenning WE, Fischmann AB, Jegasothy B, Zackheim H, et al. Preliminary evaluation of 15 chemotherapeutic agents applied topically in the treatment of mycosis fungoides. Cancer Treatment Reports 1979;63(4):619-21. - PubMed
Aviles 2015 {published data only}
-
- Aviles A, Neri N, Fernandez-Diez J, Silva L, Nambo MJ. Interferon and low doses of methotrexate versus interferon and retinoids in the treatment of refractory/relapsed cutaneous T-cell lymphoma. Hematology 2015;20(9):538–42. [PMID: 25592781] - PubMed
Bazex 1975 {published data only}
-
- Bazex A, De Mouzon J, Bazex J. Not available [Expérimentation du Dipropionate de Bétaméthazone en application locale]. Revue de Medecine de Toulouse 1975;11:1137-47.
Breneman 1991 {published data only}
-
- Breneman DL, Nartker AL, Ballman EA, Pruemer JM, Blumsack RF, Davis M, et al. Topical mechlorethamine in the treatment of mycosis fungoides. Uniformity of application and potential for environmental contamination. Journal of the American Academy of Dermatology 1991;25(6 Pt 1):1059-64. - PubMed
Cooper 1994 {published data only}
-
- Cooper IA, Wolf MM, Robertson TI, Fox RM, Matthews JP, Stone JM, et al. Randomized comparison of MACOP-B with CHOP in patients with intermediate-grade non-Hodgkin's lymphoma. The Australian and New Zealand Lymphoma Group. Journal of Clinical Oncology 1994;12(4):769-78. - PubMed
Currie 1980 {published data only}
-
- Currie VE, Kempin SJ, Young CW. Phase I trial of metoprine in patients with advanced cancer. Cancer Treatment Reports 1980;64(8-9):951-6. - PubMed
Dang 2007 {published data only}
-
- Dang NH, Pro B, Hagemeister FB, Samaniego F, Jones D, Samuels BI, et al. Phase II study of denileukin diftitox for relapsed/refractory B-Cell non-Hodgkin's lymphoma. British Journal of Haematology 2007;136(3):439-47. [PMID: ] - PubMed
Doan 1958 {published data only}
-
- Doan CA, Wiseman BK, Bouroncle BA. Clinical evaluation of CB 1348 in leukemias and lymphomas. Annals of the New York Academy of Sciences 1958;68(3):979-95. - PubMed
Dueck 2010 {published data only}
-
- Dueck G, Chua N, Prasad A, Finch D, Stewart D, White D, et al. Interim report of a phase 2 clinical trial of lenalidomide for T-cell non-Hodgkin lymphoma. Cancer 2010;116(19):4541-8. - PubMed
Duvic 2010 {published data only}
-
- Duvic M, Martin A, Olsen EA, Fivenson D, Prince M. Efficacy of denileukin diftitox retreatment in patients with cutaneous T-cell lymphoma who relapsed after initial response. Blood 2010;116(21):2863.
Fawzi 2010 {published data only}
-
- Fawzi M, El Mofty M, Ramadan S, Hegazy R, Sayed S. Broadband UVA versus PUVA in the treatment of early stage mycosis fungoides: a comparative study. Melanoma Research 2010;20:e85. [DOI: 10.1097/01.cmr.0000382933.41435.dc] - DOI
Fisher 1993 {published data only}
-
- Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. New England Journal of Medicine 1993;328(14):1002-6. - PubMed
Foss 2011 {published data only}
-
- Foss F, Duvic M, Olsen EA. Predictors of complete responses with denileukin diftitox in cutaneous T-cell lymphoma. American Journal of Hematology 2011;86(7):627-30. - PubMed
Groth 1979 {published data only}
-
- Groth O, Molin L, Thomsen K. Tumour stage of mycosis fungoides treated with bleomycin and methotrexate: report from the Scandinavian mycosis fungoides study group. Acta Dermato-Venereologica 1979;59(1):59–63. [PMID: 84469] - PubMed
Heald 2003 {published data only}
-
- Heald P, Mehlmauer M, Martin AG, Crowley CA, Yocum RC, Reich SD, et al. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: Results of the phase III clinical trial. Journal of the American Academy of Dermatology 2003;49(5):801–15. [DOI: 10.1067/S0190-9622(3)01475-0] [PMID: ] - DOI - PubMed
JapicCTI‐050041 {published data only}
-
- JapicCTI-050041. Post-marketing clinical study of Ogamma 100 in patients with mycosis fungoides. www.clinicaltrials.jp/user/showCteDetailE.jsp?japicId=JapicCTI-050041 (first received 12 September 2005).
Kaung 1969 {published data only}
-
- Kaung DT, Wittington RM, Spencer H, Patno ME. Comparison of chlorambucil and streptonigrin (NSC-45383) in the treatment of malignant lymphomas. Cancer 1969;23(6):1280-3. - PubMed
Kujawska 2003 {published data only}
-
- Kujawska A, Abrou A, Fivenson DP, Lim HW. PUVA vs. PUVA and interferon alpha for treatment of mycosis fungoides. Journal of Investigative Dermatology 2003;121(1):1185.
Kuzel 2010 {published data only}
-
- Kuzel T. Correlates of capillary leak syndrome (CLS) in patients with cutaneous T-cell lymphoma (CTCL) during treatment with denileukin diftitox (DD) in a placebo (PBO)-controlled phase III trial. Journal of Clinical Oncology 2010;28(15 Suppl):e18509.
Lambert 1986 {published data only}
-
- Lambert D, Bousser AM, Dalac S, Godard W, Lepinoy D, Horiot JC. Mycosis fungoides and radiotherapy. Value of combined total skin electron therapy and whole-body photon therapy. Annales de Dermatologie et de Venereologie 1986;113(6-7):541–7. [PMID: ] - PubMed
Lansigan 2010 {published data only}
-
- Lansigan F, Foss FM. Rate of infection and immunosuppression in two phase III studies of denileukin diftitox (DD) in cutaneous T-cell lymphoma (CTCL). Journal of Clinical Oncology 2010;28(15 Suppl):e18515.
Loescher 1984 {published data only}
-
- Loescher L, Kessler J, Jones S, Meyskens F, Levine N, Sauer K. 13-Cis retinoic acid as treatment for mycosis fungoides: Clinical results of a phase II trial. Federation Proceedings 1984;43(4):3896.
Marsden 1968 {published data only}
-
- Marsden CW. Fluocinolone acetonide 0.2 per cent cream--a co-operative clinical trial. British Journal of Dermatology 1968;80(9):614-7. [PMID: ] - PubMed
Moog 2008 {published data only}
NCT00054171 {published data only}
-
- NCT00054171. Photodynamic therapy in treating patients with lymphoma or chronic lymphocytic leukemia. clinicaltrials.gov/ct2/show/NCT00054171 (first received 6 February 2003).
NCT00091208 {unpublished data only}
-
- NCT00091208. A phase I/II open label, multi-center study for the evaluation of Pf-3512676 (CPG 7909) in patients with stage Ib to Iva cutaneous T-Cell lymphoma. clinicaltrials.gov/ct2/show/NCT00091208 (first received 12 August 2002). [NCT00091208]
NCT01007448 {unpublished data only}
-
- NCT01007448. Study evaluating two dose levels of Targretin capsules in patients with refractory cutaneous T-cell lymphoma (CTCL). clinicaltrials.gov/ct2/show/NCT01007448 (first received 4 November 2009).
NCT01187446 {unpublished data only}
-
- NCT01187446. Low-dose (12 Gy) TSEBT+Vorinostat versus low-dose TSEBT monotherapy in mycosis fungoides. clinicaltrials.gov/ct2/show/NCT01187446 (first received 24 August 2010).
NCT01386398 {unpublished data only}
-
- NCT01386398. Vorinostat with or without Bortezomib in treating patients with refractory or recurrent stage IIB, stage III, or stage IV cutaneous T-Cell lymphoma. clinicaltrials.gov/ct2/show/NCT01386398 (first received 1 July 2011).
NCT01625455 {unpublished data only}
-
- NCT01625455. Effect of Neurokinin-1 Receptor (NK1R) antagonism on pruritus in patients with Sezary syndrome. clinicaltrials.gov/ct2/show/NCT01625455 (first received 21 June 2012).
Neering 1972 {published data only}
-
- Neering H, Kroon HV, Roeleveld CG. Treatment of localized skin lesions with betamethasone 17-valerate and triamcinolone acetonide in alcoholic solution under occlusive dressing. A double blind comparative study. Dermatologica 1972;145(6):395-9. - PubMed
Negro‐Vilar 2007 {published data only}
-
- Negro-Vilar A, Dziewanowska Z, Groves ES, Stevens V, Zhang JK, Prince M, et al. Efficacy and safety of denileukin diftitox (Dd) in a phase III, double-blind, placebo-controlled study of CD25+ patients with cutaneous T-cell lymphoma (CTCL). Journal of Clinical Oncology 2007;25(18 Suppl):8026.
O'Neill 2013 {published data only}
-
- O'Neill B, Geskin L, Story S. Novel combination therapy for the treatment of cutaneous T-cell lymphoma. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB147.
Olsen 1986 {published and unpublished data}
-
- Olsen EA, Diab NG. Interferon alfa-2A in the treatment of cutaneous T cell lymphoma. Journal of Investigative Dermatology 1986;86(4):498. - PubMed
Pan 2007 {published data only}
-
- Pan Y, Prince HM, Ellis L, Culver K. LBH589, a novel deacetylase inhibitor (DACi), in the treatment of cutaneous T-cell lymphoma (CTCL): changes in tumor gene expression profiles related to clinical response after therapy. In: 11th World Congress on Cancers of the Skin. 2007 June 8-11. Amsterdam, The Netherlands. 2007:Abstract 00172.
Peugeot 1995 {published data only}
-
- Peugeot RL. Clinical efficacy of a novel purine nucleoside phosphorylase inhibitor (BCX-34) in the treatment of cutaneous T-cell lymphoma. Journal of Investigative Dermatology 1995;104(4):563.
Plettenberg 2001 {published data only}
-
- Plettenberg H, Stege H, Megahed T, Ruzicka T, Hosokawa Y, Tsuji T, et al. UVA1-Phototherapie versus PUVA-Photochemotherapie in der Behandlung von Patienten mit kutanem T-Zell-Lymphom (CTCL). Zeitschrift für Hautkrankheiten 2001;76(Suppl 1):S96.
Prince 2010 {published data only}
-
- Prince HM, Duvic M, Martin A, Sterry W, Assaf C, Sun Y, et al. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous T-cell lymphoma. Journal of Clinical Oncology 2010;28(11):1870-7. [PMID: ] - PubMed
Schrag 1997 {published data only}
-
- Schrag H-J, Dippel E, Goerdt S, Orfanos CE. Extracorporal photophoresis (ECP) + Interferon-alpha 2a vs. ECP in patients with cutaneous T-cell lymphoma. Evaluation using a new score (CTCL-SI). Der Hautarzt 1997;48(Suppl):S96.
Serri 1990 {published data only}
-
- Serri F, De Simone C, Venier A, Rusciani L, Marchetti F. Combination of retinoids and PUVA (Re-PUVA) in the treatment of cutaneous T cell lymphomas. Current Problems in Dermatology 1990;19:252-7. [PMID: ] - PubMed
Shi 2015 {published data only}
-
- Shi Y, Dong M, Zhu J, Zhou D, Huang H, Tu P, et al. Phase II study of chidamide, a new subtype-selective oral histone deacetylase inhibitor, in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood 2015;126(23):1513.
Simon 2010 {published data only}
-
- Simon A, Peoch M, Casassus P, Deconinck E, Colombat P, Desablens B, et al. Upfront VIP-reinforced-ABVD (VIP-rABVD) is not superior to CHOP/21 in newly diagnosed peripheral T cell lymphoma. Results of the randomized phase III trial GOELAMS-LTP95. British Journal of Haematology 2010;151(2):159-66. [PMID: ] - PubMed
Thomsen 1977 {published data only (unpublished sought but not used)}
-
- Thomsen K. Scandinavian mycosis fungoides study group. Bulletin du Cancer 1977;64(2):287-90. [PMID: ] - PubMed
Thomsen 1979 {published data only}
-
- Thomsen K. Scandinavian mycosis fungoides trial. Cancer Treatment Reports 1979;63(4):709–11. [PMID: ] - PubMed
Thomsen 1989 {published data only}
-
- Thomsen K, Hammar H, Molin L, Volden G. Retinoids plus PUVA (RePUVA) and PUVA in mycosis fungoides, plaque stage. Acta Dermato-Venereologica 1989;69(6):536-8. - PubMed
Touraine 1978 {published data only}
-
- Touraine R, Revuz J. Topical chemotherapy in mycosis fungoides [Chimiotherapie locale du mycosis fongoide: Dermatologica]. Dermatologica 1978;157(6):397-406. - PubMed
Wain 2005 {published data only}
-
- Wain EM, Whittaker SJ, Russell-Jones R. A randomized, open, crossover study to compare the efficacy of extracorporeal photopheresis with methotrexate in the treatment of erythrodermic primary cutaneous T-cell lymphoma. British Journal of Dermatology 2005;153(Suppl 1):10.
Wiernik 1998 {published data only}
-
- Wiernik PH, Moore DF, Bennett JM, Vogl SE, Harris JE, Luger S, et al. Phase II study of mitoguazone, cyclophosphamide, doxorubicin, vincristine and prednisone for patients with diffuse histologic subtypes of non-Hodgkin's lymphoma: an Eastern Cooperative Oncology Group Study (PE481). Leukemia & Lymphoma 1998;30(5-6):601-7. - PubMed
Wilson 1995 {published data only}
-
- Wilson LD, Licata AL, Braverman IM, Edelson RL, Heald PW, Feldman AM, et al. Systemic chemotherapy and extracorporeal photochemotherapy for T3 and T4 cutaneous T-cell lymphoma patients who have achieved a complete response to total skin electron beam therapy. International Journal of Radiation Oncology, Biology, Physics 1995;32(4):987–95. [PMID: ] - PubMed
Zubrod 1960 {published data only}
-
- Zubrod CG, Schneiderman M, Frei E, Brindley C, Gold GL, Shnider B, et al. Appraisal of methods for the study of chemotherapy of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophosphoramide. Journal of Chronic Diseases 1960;11(1):7-33.
References to studies awaiting assessment
Bashey 2014 {published data only}
-
- Bashey S, Tavallaee M, Duvic M, Dabaja B, Talpur R, Wilson L, et al. A multicenter, open-label, randomized, phase I/II study evaluating the safety and efficacy of low-dose (12 Gy) total skin electron beam therapy (TSEBT) combined with vorinostat versus low-dose TSEBT monotherapy in patients with mycosis fungoides. Journal of Investigative Dermatology 2014;134(Suppl 1):S104. [DOI: ]
Kim 2014 {published data only}
-
- Kim YH, Krathen M, Duvic M, Wong H, Porcu P, Tacastacas J, et al. A phase 1b study in cutaneous T-cell lymphoma (CTCL) with the novel topically applied skin-restricted histone deacteylase inhibitor (HDAC-i) SHP-141. Journal of Clinical Oncology 2014;32(15 Suppl):8525.
-
- Kim YH, Krathen MS, Duvic M, Wong H, Porcu P, Tacastacas J, et al. Tolerability and encouraging clinical activity of SHP-141, a topical skin-restricted HDAC inhibitor, in a phase 1B study in cutaneous t-cell lymphoma. Journal of Investigative Dermatology 2014;134(Suppl 1):S93.
-
- NCT01433731. Safety, pharmacodynamics (PD), pharmacokinetics (PK) study of SHP141 in 1A, 1B, or 2A cutaneous T-cell lymphoma (CTCL). clinicaltrials.gov/ct/show/NCT01433731 (first received 14 September 2011).
References to ongoing studies
NCT01738594 {published data only}
-
- NCT01738594. Dose-escalation trial of carfilzomib with and without Romidepsin in cutaneous T-cell lymphoma. clinicaltrials.gov/ct2/show/NCT01738594 (first received 30 November 2012).
NCT02213861 {published data only}
-
- Duvic M, Kim YH, LeBoeuf NR, Porcu P, Hastings J, Bassuner J, et al. A phase 2 randomized study of SHAPE Gel (SHP-141) in patients with early-stage cutaneous T-cell lymphoma: Interim results. Journal of Clinical Oncology 2016;34(15 Suppl):7562. [DOI: 10.1200/JCO.2016.34] - DOI
-
- NCT02213861. Efficacy, safety and tolerability study of SHAPE in IA, IB or IIA cutaneous T-cell lymphoma. clinicaltrials.gov/ct2/show/NCT02213861 (first received 12 August 2014).
NCT02301494 {published data only}
-
- NCT02301494. Effectiveness of imiquimod topical cream in early stage cutaneous T-cell lymphoma (CTCL). clinicaltrials.gov/ct2/show/NCT02301494 (first received 26 November 2014).
NCT02323659 {published data only}
-
- NCT02323659. Comparison of methotrexate versus interferon-alfa 2b in patients with primary cutaneous T-cell lymphomas. clinicaltrials.gov/ct2/show/NCT02323659 (first received 23 December 2014).
NCT02448381 {published data only}
-
- NCT02448381. FLASH [Fluorescent Light Activated Synthetic Hypericin] clinical study: topical SGX301 (synthetic hypericin) for the treatment of cutaneous T-cell lymphoma (mycosis fungoides). clinicaltrials.gov/ct2/show/NCT02448381 (first received 19 May 2015).
NCT02811783 {published data only}
-
- NCT02811783. Naloxone hydrochloride study for relief of pruritus in patients with MF or SS forms of CTCL. clinicaltrials.gov/ct2/show/NCT02811783 (first received 23 June 2016).
NCT02943642 {published data only}
-
- NCT02943642. Safety and effectiveness of A-dmDT390-bisFv(UCHT1) fusion protein in subjects with mycosis fungoides (Resimmune®). clinicaltrials.gov/ct2/show/NCT02943642 (first received 25 October 2016).
NCT02953301 {published data only}
-
- NCT02953301. Resminostat for maintenance treatment of patients with advanced stage mycosis fungoides (MF) or Sézary syndrome (SS) (RESMAIN). clinicaltrials.gov/ct2/show/NCT02953301 (first received 2 November 2016).
-
- Stadler R, Scarisbrick J, Knobler R, Quaglino P, Borgmann M, Orlovius M, et al. A multicentre, double blind, randomised, placebo controlled, Phase II trial to evaluate Resminostat for maintenance treatment of patients with advanced stage (Stage IIB-IVB) mycosis fungoides (MF) or Sezary Syndrome (SS) that have achieved disease control with systemic therapy-the RESMAIN Study. Journal of the European Academy of Dermatology and Venereology 2017;31(Suppl 3):50.
-
- Stadler R, Scarisbrick J, Knobler R, Quaglino P, Borgmann M, Orlovius M, et al. A multicentre, double blind, randomised, placebo-controlled, Phase II trial to evaluate Resminostat for maintenance treatment of patients with advanced stage (Stage IIB-IVB) mycosis fungoides (MF) or Sezary Syndrome (SS) that have achieved disease control with systemic therapy: The RESMAIN Study. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2017;15(Suppl 3):74–5. [DOI: 10.1111/ddg.13300] - DOI
NCT03011814 {published data only}
-
- NCT03011814. Durvalumab with or without lenalidomide in treating patients with relapsed or refractory cutaneous or peripheral T cell lymphoma. clinicaltrials.gov/ct2/show/NCT03011814 (first received 5 January 2017).
NCT03292406 {published data only}
-
- NCT03292406. A safety, efficacy and pharmacokinetics study of CD11301 for the treatment of cutaneous T-cell lymphoma (CTCL) (CTCL). clinicaltrials.gov/ct2/show/NCT03292406 (first received 25 September 2017).
NCT03454945 {published data only}
-
- NCT03454945. Efficacy of doxycycline in the treatment of early stages of mycosis fungoides: A randomized controlled trial. clinicaltrials.gov/ct2/show/NCT03454945 (first received 6 March 2018).
NCT03713320 {published data only}
-
- NCT03713320. SOLAR: A phase 2, randomized, open-label, parallel-group, active comparator, multi-center study to investigate the efficacy and safety of cobomarsen (MRG-106) in subjects with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) subtype. clinicaltrials.gov/ct2/show/NCT03713320 (first received 17 October 2018).
UMIN000029537 {published data only}
-
- UMIN000029537. Efficacy and safety of Targretin capsule 75-mg alone or in combination with phototherapy in Japanese patients with cutaneous T-cell lymphomas. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000033748 (date first received 16 October 2017).
Additional references
Abbott 2009
-
- Abbott RA, Whittaker SJ, Morris SL, Russell-Jones R, Hung T, Bashir SJ, et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. British Journal of Dermatology 2009;160(6):1299-307. - PubMed
Agar 2010
-
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. Journal of Clinical Oncology 2010;28(31):4730-9. [PMID: ] - PubMed
Akpek 1999
-
- Akpek G, Koh HK, Bogen S, O'Hara C, Foss FM. Chemotherapy with etoposide, vincristine, doxorubicin, bolus cyclophosphamide, and oral prednisone in patients with refractory cutaneous T-cell lymphoma. Cancer 1999;86(7):1368-76. - PubMed
Aronsson 1982
-
- Aronsson A, Jonsson N, Tegner E. Transient lymphomatoid papulosis in mycosis fungoides. Acta Dermato-Venereologica 1982;62(6):529-31. - PubMed
Avarbock 2008
-
- Avarbock AB, Loren AW, Park JY, Junkins-Hopkins JM, Choi J, Litzky LA, et al. Lethal vascular leak syndrome after denileukin diftitox administration to a patient with cutaneous gamma/delta T-cell lymphoma and occult cirrhosis. American Journal of Hematology 2008;83(7):593-5. - PubMed
Bernstein 1989
-
- Bernstein L, Deapen D, Ross RK. Mycosis fungoides. JAMA 1989;261(13):1882. [PMID: ] - PubMed
Bohmeyer 2003
-
- Bohmeyer J, Stadler R, Kremer A, Nashan D, Muche M, Gellrich S, et al. Bexarotene--an alternative therapy for progressive cutaneous T-cell lymphoma? First experiences. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2003;1(10):785-9. - PubMed
Bradford 2009
Braverman 1987
-
- Braverman IM, Yager NB, Chen M, Cadman EC, Hait WN, Maynard T. Combined total body electron beam irradiation and chemotherapy for mycosis fungoides. Journal of the American Academy of Dermatology 1987;16(1 Pt 1):45-60. - PubMed
Bunn 1979
-
- Bunn PA Jr, Lamberg SI. Report of the committee on staging and classification of cutaneous T-cell lymphomas. Cancer Treatment Reports 1979;63(4):725-8. [PMID: ] - PubMed
Campo‐Voegeli 1998
-
- Campo-Voegeli A, Estrach T, Marti RM, Corominas N, Tuset M, Mascaró JM. Acrocyanosis induced by interferon alpha(2a). Dermatology 1998;196(3):361-3. - PubMed
Carson 2014
Cella 1993
-
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11(3):570-9. [PMID: ] - PubMed
Cerroni 2018
Chan 2004
-
- Chan AW, Hróbjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457-65. - PubMed
Corbin 2017
Criscione 2007
-
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Archives of Dermatology 2007;143(7):854-9. [PMID: ] - PubMed
Daughters 1973
-
- Daughters D, Zackheim H, Maibach H. Urticaria and anaphylactoid reactions after topical application of mechlorethamine. Archives of Dermatology 1973;107(3):429-30. - PubMed
Dekkers 2010
-
- Dekkers OM, Elm E, Algra A, Romijn JA, Vandenbroucke JP. How to assess the external validity of therapeutic trials: a conceptual approach. International Journal of Epidemiology 2010;39(1):89-94. [PMID: ] - PubMed
Desai 1988
-
- Desai KR, Pezner RD, Lipsett JA, Vora NL, Luk KH, Wong JY, et al. Total skin electron irradiation for mycosis fungoides: relationship between acute toxicities and measured dose at different anatomic sites. International Journal of Radiation Oncology, Biology, Physics 1988;15(3):641-5. - PubMed
Duarte 2010
-
- Duarte R, Canals C, Onida F, Gabriel I, Arranz R, Arcese W, et al. Allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sézary syndrome: a retrospective analysis of the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation.. Journal of Clinical Oncology 2010;28(29):4492-9. [PMID: 20697072] - PubMed
Dueck 2010a
-
- Dueck G, Chua N, Prasad A, Finch D, Stewart D, White D, et al. Interim report of a phase 2 clinical trial of lenalidomide for T-cell non-Hodgkin lymphoma. Cancer 2010;116(19):4541-8. - PubMed
Dummer 2008
-
- Dummer R, Dreyling M. Primary cutaneous lymphoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Annals of Oncology 2008;19(Suppl 2):ii72-6. [PMID: ] - PubMed
Dummer 2012
-
- Dummer R, Quaglino P, Becker JC, Hasan B, Karrasch M, Whittaker S, et al. Prospective international multicenter phase II trial of intravenous pegylated liposomal doxorubicin monochemotherapy in patients with stage IIB, IVA, or IVB advanced mycosis fungoides: final results from EORTC 21012. Journal of Clinical Oncology 2012;30(33):4091-7. - PubMed
Duvic 2001b
-
- Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: multinational phase II-III trial results. Journal of Clinical Oncology 2001;19(9):2456-71. - PubMed
Duvic 2002
-
- Duvic M, Kuzel TM, Olsen EA, Martin AG, Foss FM, Kim YH, et al. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ONTAK). Clinical Lymphoma 2002;2(4):222-8. - PubMed
Duvic 2013
-
- Duvic M, Martin AG, Olsen EA, Fivenson DP, Prince HM. Efficacy and safety of denileukin diftitox retreatment in patients with relapsed cutaneous T-cell lymphoma. Leukemia & Lymphoma 2013;54(3):514-9. - PubMed
Duvic 2015
Elder 2018
-
- Elder DE, Massi D, Scolyer RA, Willemze R. WHO Classification of Skin Tumours (4th edition). IARC Press, 2018. [ISBN-13 9789283224402]
Foss 2001
-
- Foss FM, Bacha P, Osann KE, Demierre MF, Bell T, Kuzel T. Biological correlates of acute hypersensitivity events with DAB(389)IL-2 (denileukin diftitox, ONTAK) in cutaneous T-cell lymphoma: decreased frequency and severity with steroid premedication. Clinical Lymphoma 2001;1(4):298-302. - PubMed
Gilson 2019
-
- Gilson D, Whittaker SJ, Child FJ, Scarisbrick JJ, Illidge TM, Parry EJ, et al. British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018. British Journal of Dermatology 2019;180(3):496-526. [PMID: ] - PubMed
Goday 1990
-
- Goday JJ, Aguirre A, Ratón JA, Díaz-Pérez JL. Local bullous reaction to topical mechlorethamine (mustine). Contact Dermatitis 1990;22(5):306-7. - PubMed
GRADE pro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 29 March 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Grant 2009
-
- Grant B. Biotech Baddies. The Scientist 2009;23(4):48.
Grunnet 1976
-
- Grunnet E. Contact urticaria and anaphylactoid reaction induced by topical application of nitrogen mustard. British Journal of Dermatology 1976;94(1):101-3. - PubMed
Guilhou 1980
-
- Guilhou JJ, Barnéon G, Malbos S, Peyron JL, Michel B, Meynadier J. Perforating follicular mucinosis and immediate hypersensitivity to mechlorethamine in a patient with mycosis fungoides. Annales de Dermatologie et de Venereologie 1980;107(1-2):59-62. - PubMed
Guyatt 2008
Hanley 1983
-
- Hanley JA, Lippman-Hand A. If nothing goes wrong, is everything all right? Interpreting zero numerators. JAMA 1983;249(13):1743-5. [PMID: ] - PubMed
Herrmann 1995
-
- Herrmann JJ, Roenigk HH Jr, Hurria A, Kuzel TM, Samuelson E, Rademaker AW, et al. Treatment of mycosis fungoides with photochemotherapy (PUVA): long-term follow-up. Journal of the American Academy of Dermatology 1995;33(2 Pt 1):234-42. - PubMed
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hollis 1999
Hoppe 1987
-
- Hoppe RT, Abel EA, Deneau DG, Price NM. Mycosis fungoides: management with topical nitrogen mustard. Journal of Clinical Oncology 1987;5(11):1796-803. - PubMed
Humme 2014
-
- Humme D, Nast A, Erdmann R, Vandersee S, Beyer M. Systematic review of combination therapies for mycosis fungoides. Cancer Treatment Reviews 2014;40(8):927-33. [PMID: ] - PubMed
Hwang 2008
-
- Hwang ST, Janik JE, Jaffe ES, Wilson WH. Mycosis fungoides and Sézary syndrome. Lancet 2008;371(9616):945-57. [PMID: ] - PubMed
Hüsken 2012
-
- Hüsken AC, Tsianakas A, Hensen P, Nashan D, Loquai C, Beissert S, et al. Comparison of pegylated interferon α-2b plus psoralen PUVA versus standard interferon α-2a plus PUVA in patients with cutaneous T-cell lymphoma. Journal of the European Academy of Dermatology and Venereology : JEADV 2012;26(1):71-8. - PubMed
Jaffe 2001
-
- Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics: Tumours of Hematopoetic and Lymphoid Tissues (World Health Organization Classification of Tumours). Lyon: IARC Press, 2001.
Jüni 2001
Kairouani 2012
Kim 1996
-
- Kim YH, Jensen RA, Watanabe GL, Varghese A, Hoppe RT. Clinical stage IA (limited patch and plaque) mycosis fungoides. A long-term outcome analysis. Archives of Dermatology 1996;132(11):1309-13. [PMID: ] - PubMed
Kim 2003
-
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: Clinical prognostic factors and risk for disease progression. Archives of Dermatology 2003;139(7):857-66. [PMID: ] - PubMed
Kim 2015
-
- Kim YH, Tavallaee M, Sundram U, Salva KA, Wood GS, Li S, et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and Sézary syndrome with variable CD30 expression level: a multi-institution collaborative project. Journal of Clinical Oncology 2015;33(32):3750–8. - PMC - PubMed
Korgavkar 2013
Korpusik 2007
-
- Korpusik D, Homey B, Stege H, Bruch-Gerharz D, Schulte KW. Mycosis fungoides: Complications of long-term treatment with PUVA and ECP [Mycosis fungoides: Komplikationen einer Langzeitbehandlung mit PUVA und ECP]. Hautarzt 2007;58(4):298-9. [EMBASE: 46623430] - PubMed
Kütting 1997
-
- Kütting B, Böhm M, Luger TA, Bonsmann G. Oropharyngeal lichen planus associated with interferon-alpha treatment for mycosis fungoides: a rare side-effect in the therapy of cutaneous lymphomas. British Journal of Dermatology 1997;137(5):836-7. - PubMed
Legroux‐Crespel 2003
-
- Legroux-Crespel E, Lafaye S, Mahé E, Picard-Dahan C, Crickx B, Sassolas B, et al. Seizures during interferon alpha therapy: three cases in dermatology. Annales de Dermatologie et de Venereologie 2003;130(2 Pt 1):202-4. - PubMed
Lessin 2011
-
- Lessin S, Duvic M, Guitart J, Pandya A, Strober B, Olsen E, et al. Positive results of a pivotal phase ii trial testing a manufactured 0.02% Mechlorethamine gel in mycosis fungoides (mf). Journal of Investigative Dermatology 2011;131(Suppl 1):S84. [CENTRAL: CN-00843792]
Lorincz 1996
-
- Lorincz AL. Cutaneous T-cell lymphoma (mycosis fungoides). Lancet 1996;347(9005):871-6. [PMID: ] - PubMed
McCann 2012
-
- McCann S, Akilov OE, Geskin L. Adverse effects of denileukin diftitox and their management in patients with cutaneous T-cell lymphoma. Clinical Journal of Oncology Nursing 2012;16(5):E164-72. - PubMed
Micaily 1983
-
- Micaily B, Vonderheid EC, Brady LW. Combined moderate dose electron beam radiotherapy and topical chemotherapy for cutaneous T-Cell lymphoma. International Journal of Radiation Oncology, Biology, Physics 1983;9(4):475-9. - PubMed
Miettinen 1985
-
- Miettinen O, Nurminen M. Comparative analysis of two rates. Statistics in Medicine 1985;4(2):213-26. [PMID: ] - PubMed
Mna 2016
-
- Mna AB, Souissi A, Halouani S, El Euch D, Zahani A, Kchir N, et al. Methotrexate-induced necrolysis in tumoral-stage mycosis fungoides: a challenging diagnosis. Dermatology Online Journal 2016;22(1):16. - PubMed
Moher 1995
-
- Moher D, Jadad AR, Nichol G, Penman M, Tugwell P, Walsh S. Assessing the quality of randomized controlled trials: An annotated bibliography of scales and checklists. Controlled Clinical Trials 1995;16(1):62-73. [PMID: ] - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [PMID: ] - PubMed
Molin 1979
-
- Molin L, Thomsen K, Volden G. Mycosis fungoides plaque stage treated with topical nitrogen mustard with and without attempts at tolerance induction: report from the Scandinavian mycosis fungoides study group. Acta Dermato-Venereologica 1979;59(1):64-8. [PMID: ] - PubMed
Molin 1981
-
- Molin L, Thomsen K, Volden G, Groth O. Photochemotherapy (PUVA) in the pretumour stage of mycosis fungoides: a report from the Scandinavian Mycosis Fungoides Study Group. Acta Dermatovenereologica (Stockholm) 1981;61(1):47-51. - PubMed
Morales 2000
-
- Morales Suárez-Varela MM, Llopis González A, Marquina Vila A, Bell J. Mycosis fungoides: Review of epidemiological observations. Dermatology 2000;201(1):21-8. [PMID: ] - PubMed
Nath 2014
Newman 1997
-
- Newman JM, Rindler JM, Bergfeld WF, Brydon JK. Stevens-Johnson syndrome associated with topical nitrogen mustard therapy. Journal of the American Academy of Dermatology 1997;36(1):112-4. - PubMed
Olsen 2007
-
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007;110(6):1713-22. [PMID: ] - PubMed
Olsen 2011
-
- Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and Sezary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. Journal of Clinical Oncology 2011;29(18):2598-607. [PMID: ] - PMC - PubMed
Olsen 2016
-
- Olsen EA, Hodak E, Anderson T, Carter JB, Henderson M, Cooper K, et al. Guidelines for phototherapy of mycosis fungoides and Sézary syndrome: A consensus statement of the United States Cutaneous Lymphoma Consortium. Journal of the American Academy of Dermatology 2015;74(1):27-58. [DOI: 10.1016/j.jaad.2015.09.033] [PMID: ] - DOI - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration., 2014.
Rupoli 2005
-
- Rupoli S, Goteri G, Pulini S, Filosa A, Tassetti A, Offidani M, et al. Long-term experience with low-dose interferon-alpha and PUVA in the management of early mycosis fungoides. European Journal of Haematology 2005;75(2):136-45. - PubMed
Sanchez 1977
-
- Sánchez Yus E, Surárez Martín E. Contact urticaria and anaphylactic reactions induced by topical application of nitrogen mustard. Actas Dermo-Sifiliograficas 1977;68(12):39-44. - PubMed
Sauder 2003
-
- Sauder DN. Imiquimod: modes of action. British Journal of Dermatology 2003;149(s66):5-8. - PubMed
Sausville 1988
-
- Sausville EA, Eddy JL, Makuch RW, Fischmann AB, Schechter GP, Matthews M, et al. Histopathologic staging at initial diagnosis of mycosis fungoides and the Sézary syndrome. Definition of three distinctive prognostic groups. Annals of Internal Medicine 1988;109(5):372-82. [PMID: ] - PubMed
Schlaak 2013
-
- Schlaak M, Pickenhain J, Theurich S, Skoetz N, Bergwelt-Baildon M, Kurschat P. Allogeneic stem cell transplantation versus conventional therapy for advanced primary cutaneous T-cell lymphoma. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD008908.pub3] - DOI - PMC - PubMed
Schwartzman 1994
-
- Schwartzman R, Cidlowski J. Glucocorticoid-induced apoptosis of lymphoid cells. International Archives of Allergy and Immunology 1994;105(4):347-54. [PMID: ] - PubMed
Simoni 1987
-
- Simoni R, Cavalieri R, Coppola G, Ricciotti L, De Pità O, Criscuolo D, et al. Recombinant leukocyte interferon alfa-2a in the treatment of mycosis fungoides. Journal of Biological Regulators and Homeostatic Agents 1987;1(2):93-9. - PubMed
Sokolowska‐Wojdylo 2016
-
- Sokolowska-Wojdylo M, Florek A, Zaucha JM, Chmielowska E, Giza A, Knopinska-Posluszny W, et al. Polish lymphoma research group experience with bexarotene in the treatment of cutaneous T-cell lymphoma. American Journal of Therapeutics 2016;23(3):e749-56. - PubMed
Spaccarelli 2015
-
- Spaccarelli N, Rook AH. The use of interferons in the treatment of cutaneous T-cell lymphoma. Dermatologic Clinics 2015;33(4):731-45. [PMID: ] - PubMed
Spitzer 1981
-
- Spitzer WO, Dobson AJ, Hall J, Chesterman E, Levi J, Shepherd R, et al. Measuring the quality of life of cancer patients: a concise QL-index for use by physicians. Journal of Chronic Diseases 1981;34(12):585-97. [PMID: ] - PubMed
Talpur 2012
-
- Talpur R, Duvic M. Pilot study of denileukin diftitox alternate dosing regimen in patients with cutaneous peripheral T-cell lymphomas. Clinical Lymphoma, Myeloma & Leukemia 2012;12(3):180-5. - PubMed
Toumishey 2015
-
- Toumishey E, Prasad A, Dueck G, Chua N, Finch D, Johnston J, et al. Final report of a phase 2 clinical trial of lenalidomide monotherapy for patients with T-cell lymphoma. Cancer 2015;121(5):716-23. - PubMed
Trautinger 2006
-
- Trautinger F, Knobler R, Willemze R, Peris K, Stadler R, Laroche L, et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. European Journal of Cancer 2006;42(8):1014-30. [PMID: ] - PubMed
Trautinger 2017
-
- Trautinger F, Eder J, Assaf C, Bagot M, Cozzio A, Dummer R, et al. European organisation for research and treatment of cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome - Update 2017. European Journal of Cancer 2017;77:57-74. [DOI: 10.1016/j.ejca.2017.02.027] [PMID: ] - DOI - PubMed
van Doorn 2000
-
- Doorn R, Van Haselen CW, Voorst Vader PC, Geerts ML, Heule F, Rie M, et al. Mycosis fungoides: Disease evolution and prognosis of 309 Dutch patients. Archives of Dermatology 2000;136(4):504-10. [PMID: ] - PubMed
van Scott 1973
-
- Van Scott EJ, Kalmanson JD. Complete remissions of mycosis fungoides lymphoma induced by topical nitrogen mustard (HN2). Control of delayed hypersensitivity to HN2 by desensitization and by induction of specific immunologic tolerance. Cancer 1973;32(1):18-30. [PMID: ] - PubMed
Vegna 1990
-
- Vegna ML, Papa G, Defazio D, Pisani F, Coppola G, De Pità O, et al. Interferon alpha-2a in cutaneous T-cell lymphoma. European Journal of Haematology. Supplementum 1990;52:32-5. - PubMed
Verhagen 1998
-
- Verhagen AP, Vet HC, Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. Journal of Clinical Epidemiology 1998;51(12):1235-41. [PMID: ] - PubMed
Vonderheid 2006
-
- Vonderheid EC, Pena J, Nowell P. Sézary cell counts in erythrodermic cutaneous T-cell lymphoma: Implications for prognosis and staging. Leukemia & Lymphoma 2006;47(9):1841-56. [PMID: ] - PubMed
Wain 2003
-
- Wain EM, Orchard GE, Whittaker SJ, Spittle MF, Russell-Jones R. Outcome in 34 patients with juvenile-onset mycosis fungoides: A clinical, immunophenotypic, and molecular study. Cancer 2003;98(10):2282-90. [PMID: ] - PubMed
Weinstock 1988
-
- Weinstock MA, Horm JW. Mycosis fungoides in the United States. Increasing incidence and descriptive epidemiology. JAMA 1988;260(1):42-6. [PMID: ] - PubMed
Weinstock 1999
-
- Weinstock MA, Reynes JF. The changing survival of patients with mycosis fungoides: A population-based assessment of trends in the United States. Cancer 1999;85(1):208-12. [PMID: ] - PubMed
Whittaker 2003
-
- Whittaker SJ, Marsden JR, Spittle M, Russell Jones R. Joint British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous T-cell lymphomas. British Journal of Dermatology 2003;149(6):1095-107. [PMID: ] - PubMed
Willemze 1997
-
- Willemze R, Kerl H, Sterry W, Berti E, Cerroni L, Chimenti S, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood 1997;90(1):354-71. [PMID: ] - PubMed
Willemze 2005
-
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005;105(10):3768-85. [PMID: ] - PubMed
Willemze 2019
Wu 2009
Yoo 1996
-
- Yoo EK, Rook AH, Elenitsas R, Gasparro FP, Vowels BR. Apoptosis induction of ultraviolet light A and photochemotherapy in cutaneous T-cell Lymphoma: relevance to mechanism of therapeutic action. Journal Investigative Dermatology 1996;107(2):235-42. [PMID: ] - PubMed
Youssef 2013
-
- Youssef M, Mokni S, Belhadjali H, Aouem K, Moussa A, Laatiri A, et al. Cyclophosphamide-induced generalised reticulated skin pigmentation: a rare presentation. International Journal of Clinical Pharmacy 2013;35(3):309-12. - PubMed
Zackheim 1996
-
- Zackheim HS, Kashani-Sabet M, Hwang ST. Low-dose methotrexate to treat erythrodermic cutaneous T-cell lymphoma: results in twenty-nine patients. Journal of the American Academy of Dermatology 1996;34(4):626-31. - PubMed
Zackheim 1999
-
- Zackheim HS, Amin S, Kashani-Sabet M, McMillan A. Prognosis in cutaneous T-cell lymphoma by skin stage: Long-term survival in 489 patients. Journal of the American Academy of Dermatology 1999;40(3):418-25. [PMID: ] - PubMed
References to other published versions of this review
Weberschock 2011
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

