A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm
- PMID: 32633718
- PMCID: PMC7410499
- DOI: 10.7554/eLife.59177
A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm
Abstract
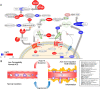
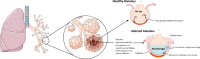
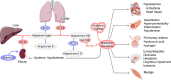
Neither the disease mechanism nor treatments for COVID-19 are currently known. Here, we present a novel molecular mechanism for COVID-19 that provides therapeutic intervention points that can be addressed with existing FDA-approved pharmaceuticals. The entry point for the virus is ACE2, which is a component of the counteracting hypotensive axis of RAS. Bradykinin is a potent part of the vasopressor system that induces hypotension and vasodilation and is degraded by ACE and enhanced by the angiotensin1-9 produced by ACE2. Here, we perform a new analysis on gene expression data from cells in bronchoalveolar lavage fluid (BALF) from COVID-19 patients that were used to sequence the virus. Comparison with BALF from controls identifies a critical imbalance in RAS represented by decreased expression of ACE in combination with increases in ACE2, renin, angiotensin, key RAS receptors, kinogen and many kallikrein enzymes that activate it, and both bradykinin receptors. This very atypical pattern of the RAS is predicted to elevate bradykinin levels in multiple tissues and systems that will likely cause increases in vascular dilation, vascular permeability and hypotension. These bradykinin-driven outcomes explain many of the symptoms being observed in COVID-19.
Keywords: COVID-19; bradykinin; computational biology; human; human biology; hyaluronic acid; medicine; pathogenesis; renin-angiotensin system; systems biology.
Plain language summary
In late 2019, a new virus named SARS-CoV-2, which causes a disease in humans called COVID-19, emerged in China and quickly spread around the world. Many individuals infected with the virus develop only mild, symptoms including a cough, high temperature and loss of sense of smell; while others may develop no symptoms at all. However, some individuals develop much more severe, life-threatening symptoms affecting the lungs and other parts of the body including the heart and brain. SARS-CoV-2 uses a human enzyme called ACE2 like a ‘Trojan Horse’ to sneak into the cells of its host. ACE2 lowers blood pressure in the human body and works against another enzyme known as ACE (which has the opposite effect). Therefore, the body has to balance the levels of ACE and ACE2 to maintain a normal blood pressure. It remains unclear whether SARS-CoV-2 affects how ACE2 and ACE work. When COVID-19 first emerged, a team of researchers in China studied fluid and cells collected from the lungs of patients to help them identify the SARS-CoV-2 virus. Here, Garvin et al. analyzed the data collected in the previous work to investigate whether changes in how the body regulates blood pressure may contribute to the life-threatening symptoms of COVID-19. The analyses found that SARS-CoV-2 caused the levels of ACE in the lung cells to decrease, while the levels of ACE2 increased. This in turn increased the levels of a molecule known as bradykinin in the cells (referred to as a ‘Bradykinin Storm’). . Previous studies have shown that bradykinin induces pain and causes blood vessels to expand and become leaky which will lead to swelling and inflammation of the surrounding tissue. In addition, the analyses found that production of a substance called hyaluronic acid was increased and the enzymes that could degrade it greatly decreased. Hyaluronic acid can absorb more than 1,000 times its own weight in water to form a hydrogel. The Bradykinin-Storm-induced leakage of fluid into the lungs combined with the excess hyaluronic acid would likely result in a Jello-like substance that is preventing oxygen uptake and carbon dioxide release in the lungs of severely affected COVID-19 patients. Therefore, the findings of Garvin et al. suggest that the Bradykinin Storm may be responsible for the more severe symptoms of COVID-19. Further experiments identified several existing medicinal drugs that have the potential to be re-purposed to treat the Bradykinin Storm. A possible next step would be to carry out clinical trials to assess how effective these drugs are in treating patients with COVID-19. In addition, understanding how SARS-Cov-2 affects the body will help researchers and clinicians identify individuals who are most at risk of developing life-threatening symptoms.
Conflict of interest statement
MG, CA, JM, EP, AW, BA, AM, AJ, BA, DJ No competing interests declared
Figures

References
-
- Adachi T, Chong J-M, Nakajima N, Sano M, Yamazaki J, Miyamoto I, Nishioka H, Akita H, Sato Y, Kataoka M, Katano H, Tobiume M, Sekizuka T, Itokawa K, Kuroda M, Suzuki T. Clinicopathologic and immunohistochemical findings from autopsy of patient with COVID-19, Japan. Emerging Infectious Diseases. 2020;26:201353. doi: 10.3201/eid2609.201353. - DOI - PMC - PubMed
-
- Alipio M. Vitamin D supplementation could possibly improve clinical outcomes of patients infected with Coronavirus-2019 (COVID-2019) SSRN Electronic Journal. 2020;2:100051. doi: 10.2139/ssrn.3571484. - DOI
-
- Arendse LB, Danser AHJ, Poglitsch M, Touyz RM, Burnett JC, Llorens-Cortes C, Ehlers MR, Sturrock ED. Novel therapeutic approaches targeting the Renin-Angiotensin system and associated peptides in hypertension and heart failure. Pharmacological Reviews. 2019;71:539–570. doi: 10.1124/pr.118.017129. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

