Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease
- PMID: 32633856
- PMCID: PMC7386785
- DOI: 10.1002/14651858.CD013665
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease
Update in
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2021 Feb 23;2(2):CD013665. doi: 10.1002/14651858.CD013665.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 20;5:CD013665. doi: 10.1002/14651858.CD013665.pub3. PMID: 33620086 Free PMC article. Updated.
Abstract
Background: Some people with SARS-CoV-2 infection remain asymptomatic, whilst in others the infection can cause mild to moderate COVID-19 disease and COVID-19 pneumonia, leading some patients to require intensive care support and, in some cases, to death, especially in older adults. Symptoms such as fever or cough, and signs such as oxygen saturation or lung auscultation findings, are the first and most readily available diagnostic information. Such information could be used to either rule out COVID-19 disease, or select patients for further diagnostic testing.
Objectives: To assess the diagnostic accuracy of signs and symptoms to determine if a person presenting in primary care or to hospital outpatient settings, such as the emergency department or dedicated COVID-19 clinics, has COVID-19 disease or COVID-19 pneumonia.
Search methods: On 27 April 2020, we undertook electronic searches in the Cochrane COVID-19 Study Register and the University of Bern living search database, which is updated daily with published articles from PubMed and Embase and with preprints from medRxiv and bioRxiv. In addition, we checked repositories of COVID-19 publications. We did not apply any language restrictions.
Selection criteria: Studies were eligible if they included patients with suspected COVID-19 disease, or if they recruited known cases with COVID-19 disease and controls without COVID-19. Studies were eligible when they recruited patients presenting to primary care or hospital outpatient settings. Studies including patients who contracted SARS-CoV-2 infection while admitted to hospital were not eligible. The minimum eligible sample size of studies was 10 participants. All signs and symptoms were eligible for this review, including individual signs and symptoms or combinations. We accepted a range of reference standards including reverse transcription polymerase chain reaction (RT-PCR), clinical expertise, imaging, serology tests and World Health Organization (WHO) or other definitions of COVID-19.
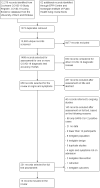
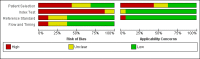
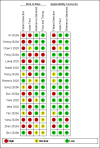
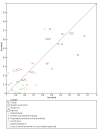
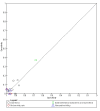
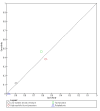
Data collection and analysis: Pairs of review authors independently selected all studies, at both title and abstract stage and full-text stage. They resolved any disagreements by discussion with a third review author. Two review authors independently extracted data and resolved disagreements by discussion with a third review author. Two review authors independently assessed risk of bias using the QUADAS-2 checklist. Analyses were descriptive, presenting sensitivity and specificity in paired forest plots, in ROC (receiver operating characteristic) space and in dumbbell plots. We did not attempt meta-analysis due to the small number of studies, heterogeneity across studies and the high risk of bias.
Main results: We identified 16 studies including 7706 participants in total. Prevalence of COVID-19 disease varied from 5% to 38% with a median of 17%. There were no studies from primary care settings, although we did find seven studies in outpatient clinics (2172 participants), and four studies in the emergency department (1401 participants). We found data on 27 signs and symptoms, which fall into four different categories: systemic, respiratory, gastrointestinal and cardiovascular. No studies assessed combinations of different signs and symptoms and results were highly variable across studies. Most had very low sensitivity and high specificity; only six symptoms had a sensitivity of at least 50% in at least one study: cough, sore throat, fever, myalgia or arthralgia, fatigue, and headache. Of these, fever, myalgia or arthralgia, fatigue, and headache could be considered red flags (defined as having a positive likelihood ratio of at least 5) for COVID-19 as their specificity was above 90%, meaning that they substantially increase the likelihood of COVID-19 disease when present. Seven studies carried a high risk of bias for selection of participants because inclusion in the studies depended on the applicable testing and referral protocols, which included many of the signs and symptoms under study in this review. Five studies only included participants with pneumonia on imaging, suggesting that this is a highly selected population. In an additional four studies, we were unable to assess the risk for selection bias. These factors make it very difficult to determine the diagnostic properties of these signs and symptoms from the included studies. We also had concerns about the applicability of these results, since most studies included participants who were already admitted to hospital or presenting to hospital settings. This makes these findings less applicable to people presenting to primary care, who may have less severe illness and a lower prevalence of COVID-19 disease. None of the studies included any data on children, and only one focused specifically on older adults. We hope that future updates of this review will be able to provide more information about the diagnostic properties of signs and symptoms in different settings and age groups.
Authors' conclusions: The individual signs and symptoms included in this review appear to have very poor diagnostic properties, although this should be interpreted in the context of selection bias and heterogeneity between studies. Based on currently available data, neither absence nor presence of signs or symptoms are accurate enough to rule in or rule out disease. Prospective studies in an unselected population presenting to primary care or hospital outpatient settings, examining combinations of signs and symptoms to evaluate the syndromic presentation of COVID-19 disease, are urgently needed. Results from such studies could inform subsequent management decisions such as self-isolation or selecting patients for further diagnostic testing. We also need data on potentially more specific symptoms such as loss of sense of smell. Studies in older adults are especially important.
Copyright © 2020 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
Thomas Struyf: none known
Jonathan J Deeks: none known
Jacqueline Dinnes: none known
Yemisi Takwoingi: none known
Clare Davenport: none known
Mariska MG Leeflang: none known
René Spijker: the Dutch Cochrane Centre (DCC) has received grants for performing commissioned systematic reviews. In no situation, the commissioner had any influence on the results of the work.
Lotty Hooft: none known
Devy Emperador: is employed by FIND. FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Sabine Dittrich: is employed by FIND with funding from DFID and Australian Aid. FIND is a global non‐for profit product development partnership and WHO Diagnostic Collaboration Centre. It is FIND’s role to accelerate access to high quality diagnostic tools for low resource settings and this is achieved by supporting both R&D and access activities for a wide range of diseases, including COVID‐19. .FIND has several clinical research projects to evaluate multiple new diagnostic tests against published Target Product Profiles that have been defined through consensus processes. These studies are for diagnostic products developed by private sector companies who provide access to know‐how, equipment/reagents, and contribute through unrestricted donations as per FIND policy and external SAC review.
Julie Domen: none known
Sebastiaan Horn: none known
Ann Van den Bruel: none known
Figures








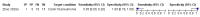




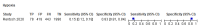





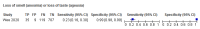

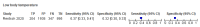
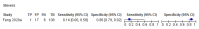
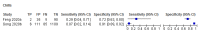
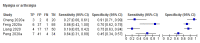
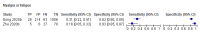


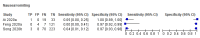

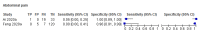




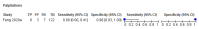
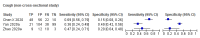
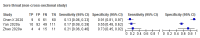

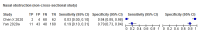
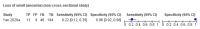

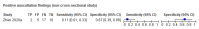


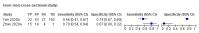
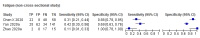

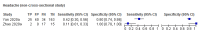
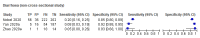
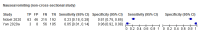

References
References to studies included in this review
Ai 2020a {published data only}
-
- Ai JW, Zhang HC, Xu T, Wu J, Zhu M, Yu YQ, et al. Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.13.20022673] - DOI
Cheng 2020a {published data only}
Chen X 2020 {published data only}
Feng 2020a {published data only}
Liang 2020 {published data only}
-
- Liang Y, Liang J, Zhou Q, Li X, Lin F, Deng Z, et al. Prevalence and clinical features of 2019 novel coronavirus disease (COVID-19) in the Fever Clinic of a teaching hospital in Beijing: a single-center, retrospective study. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.25.20027763] - DOI
Nobel 2020 {published data only}
Peng 2020a {published data only}
-
- Peng L, Liu KY, Xue F, Miao YF, Tu PA, Zhou C. Improved early recognition of coronavirus disease-2019 (COVID-19): single-center data from a Shanghai screening hospital. Archives of Iranian Medicine 2020;23(4):272-6. - PubMed
Rentsch 2020 {published data only}
-
- Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT, Skanderson M, et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54-75 years. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.09.20059964] - DOI
Song 2020b {published data only}
-
- Song CY, Xu J, He JQ, Lu YQ. COVID-19 early warning score: a multi-parameter screening tool to identify highly suspected patients. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.03.05.20031906] - DOI
Sun 2020a {published data only}
Tolia 2020 {published data only}
Wee 2020 {published data only}
-
- Wee LE, Chan YZ, Teo NY, Cherng BZ, Thien SY, Wong M, et al. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. European Archives of Oto-Rhino-Laryngology 2020 Apr 24 [Epub ahead of print]. [DOI: 10.1007/s00405-020-05999-5] - DOI - PMC - PubMed
Yan 2020a {published data only}
Yang 2020d {published data only}
Zhao 2020a {published data only}
References to studies excluded from this review
Guan 2020 {published data only}
-
- Guan, W, Ni, Z, Hu, Y, Liang, W, Ou, C, He, J, et al. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.02.06.20020974] - DOI
Soares 2020 {published data only}
-
- Soares F, Villavicencio A, Anzanello MJ, Fogliatto FS, Idiart M, Stevenson M. A novel high specificity COVID-19 screening method based on simple blood exams and artificial intelligence. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.04.10.20061036] - DOI
Song 2020 {published data only}
References to ongoing studies
ChiCTR2000029462 {published data only}
-
- ChiCTR2000029462. Study for clinical characteristics and distribution of TCM syndrome of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=48922 (first received 27 April 2020).
ChiCTR2000029734 {published data only}
-
- ChiCTR2000029734. Epidemiological investigation and clinical characteristics analysis of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=48868 (first received 27 April 2020).
ChiCTR2000029770 {published data only}
-
- ChiCTR2000029770. Study for epidemiology, diagnosis and treatment of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/hvshowproject.aspx?id=23744 (first received 27 April 2020).
ChiCTR2000029839 {published data only}
-
- ChiCTR2000029839. An observational study on the clinical characteristics, treatment and outcome of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=49439 (first received 27 April 2020).
ChiCTR2000029865 {published data only}
-
- ChiCTR2000029865. Descriptive study on the clinical characteristics and outcomes of novel coronavirus pneumonia (COVID-19) in cardiovascular patients. www.chictr.org.cn/showproj.aspx?proj=49545 (first received 27 April 2020).
ChiCTR2000029866 {published data only}
-
- ChiCTR2000029866. Early warning prediction of patients with severe novel coronavirus pneumonia (COVID-19) based on multiomics. www.chictr.org.cn/showproj.aspx?proj=49519 (first received 27 April 2020).
ChiCTR2000029959 {published data only}
-
- ChiCTR2000029959. Clinical observation and research of Severe acute respiratory syndrome coronavirus 2(COVID-19) infection in perinatal newborns. www.chictr.org.cn/showproj.aspx?proj=49636 (first received 27 April 2020).
ChiCTR 2000030096 {published data only}
-
- ChiCTR 2000030096. Study for establishment of correlation between virological dynamics and clinical features in novel coronavirus pneumonia (COVID-19). http://www.chictr.org.cn/showprojen.aspx?proj=49794 (first received 27 April 2020).
ChiCTR2000030256 {published data only}
-
- ChiCTR2000030256. Epidemiological and clinical characteristics of COVID-19: a large-scale investigation in epicenter Wuhan, China. www.chictr.org.cn/showproj.aspx?proj=50078 (first received 27 April 2020).
ChiCTR2000030327 {published data only}
-
- ChiCTR2000030327. Analysis of clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50214 (first received 27 April 2020).
ChiCTR2000030363 {published data only}
-
- ChiCTR2000030363. Novel coronavirus infected disease (COVID-19) in children: epidemiology, clinical features and treatment outcome. www.chictr.org.cn/showproj.aspx?proj=49984 (first received 27 April 2020).
ChiCTR2000030387 {published data only}
-
- ChiCTR2000030387. Clinical observation and research of multiple organs injury in severe patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50329 (first received 27 April 2020).
ChiCTR2000030464 {published data only}
-
- ChiCTR2000030464. Study for the clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50382 (first received 27 April 2020).
ChiCTR2000030491 {published data only}
-
- ChiCTR2000030491. A medical records based study for comparing differences of clinical features and outcomes of novel coronavirus pneumonia (COVID-19) patients between Sichuan Province and Wuhan City. www.chictr.org.cn/hvshowproject.aspx?id=23102 (first received 27 April 2020).
ChiCTR2000030519 {published data only}
-
- ChiCTR2000030519. Study for the clinical characteristics and digestive system damage of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50604 (first received 27 April 2020).
ChiCTR2000030544 {published data only}
-
- ChiCTR2000030544. Study for the risk factors of critically ill patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50134 (first received 27 April 2020).
ChiCTR 2000030679 {published data only}
-
- ChiCTR 2000030679. Cohort study of novel coronavirus infected diseases (COVID-19) in children. www.chictr.org.cn/hvshowproject.aspx?id=23417 (first received 27 April 2020).
ChiCTR2000030707 {published data only}
-
- ChiCTR2000030707. Retrospective study on novel coronavirus pneumonia (COVID-19) in Tibetan Plateau. www.chictr.org.cn/showproj.aspx?proj=50160 (first received 27 April 2020).
ChiCTR2000030722 {published data only}
-
- ChiCTR2000030722. Auscultatory characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50338 (first received 27 April 2020).
ChiCTR2000030739 {published data only}
-
- ChiCTR2000030739. Exploration of the clinical characteristics of patients with novel coronavirus pneumonia (COVID-19) and its differences from patients with severe influenza A and MERS. www.chictr.org.cn/showproj.aspx?proj=50896 (first received 27 April 2020).
ChiCTR2000030755 {published data only}
-
- ChiCTR2000030755. A medical records based study for characteristics, prognosis of elderly patients with novel coronavirus pneumonia (COVID-19) in Wuhan area. www.chictr.org.cn/hvshowproject.aspx?id=23554 (first received 27 April 2020).
ChiCTR2000030778 {published data only}
-
- ChiCTR2000030778. A medical records based study for epidemic and clinical features of novel coronavirus pneumonia (COVID-19) in Ningbo First Hospital. www.chictr.org.cn/hvshowproject.aspx?id=23642 (first received 27 April 2020).
ChiCTR2000030784 {published data only}
-
- ChiCTR2000030784. A study for clinical characteristics of novel coronavirus pneumonia (COVID-19) patients follow-up in Guangxi. www.chictr.org.cn/showproj.aspx?proj=50307 (first received 27 April 2020).
ChiCTR2000030796 {published data only}
-
- ChiCTR2000030796. Clinical characteristics and treatment of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=50991 (first received 27 April 2020).
ChiCTR 2000030798 {published data only}
-
- ChiCTR 2000030798. A medical records based study for clinical characteristics of novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/hvshowproject.aspx?id=23687 (first received 27 April 2020).
ChiCTR2000030803 {published data only}
-
- ChiCTR2000030803. Collection and analysis of clinical data in severe and critically ill patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=51007 (first received 27 April 2020).
ChiCTR2000030807 {published data only}
-
- ChiCTR2000030807. Clinical characteristics and prognosis of cancer patients with novel coronavirus pneumonia (COVID-19). www.chictr.org.cn/showproj.aspx?proj=51019 (first received 27 April 2020).
ChiCTR2000030818 {unpublished data only}
-
- ChiCTR2000030818. A medical records based study for the value of Lymphocyte subsets in the diagnose and treatment. http://www.chictr.org.cn/hvshowproject.aspx?id=23742 (first received 27 April 2020).
ChiCTR2000030819 {published data only (unpublished sought but not used)}
-
- ChiCTR2000030819. Retrospective analysis of digestive system symptoms in 600 cases of novel coronavirus pneumonia (COVID-19) in Guanggu district, Wuhan. www.chictr.org.cn/showproj.aspx?proj=51039 2020.
ChiCTR2000030834 {published data only}
-
- ChiCTR2000030834. Epidemiological characteristics and antibody levels of novel coronavirus pneumonia (COVID-19) of pediatric medical staff working in quarantine area. www.chictr.org.cn/showproj.aspx?proj=51047 (frist received 27 April 2020).
ChiCTR2000030854 {published data only}
-
- ChiCTR2000030854. A clinical multicenter study for the occurrence, development and prognosis of novel coronavirus pneumonia (COVID-19). http://www.chictr.org.cn/showprojen.aspx?proj=51083 (first received 27 April 2020).
ChiCTR2000030858 {published data only}
-
- ChiCTR2000030858. Clinical characteristics and outcomes of 483 mild patients with novel coronavirus pneumonia (COVID-19) in Wuhan, China during the outbreak: a single-center, retrospective study from the mobile cabin hospital. www.chictr.org.cn/showproj.aspx?proj=51097 (first received 27 April 2020).
ChiCTR2000030863 {published data only}
-
- ChiCTR2000030863. Clinical and CT imaging characteristics of novel coronavirus pneumonia (COVID-19): an multicenter cohort study. www.chictr.org.cn/showproj.aspx?proj=50767 (first received 27 April 2020).
NCT04270383 {published data only}
-
- NCT04270383. Clinical characteristics and long-term prognosis of 2019-nCoV infection in children. clinicaltrials.gov/ct2/show/NCT04270383 (first received 17 February 2020).
NCT04279782 {published data only}
-
- NCT04279782. Clinical features of suspected and confirmed patients of 2019 novel coronavirus infection. www.clinicaltrials.gov/ct2/show/NCT04279782 (first received 27 April 2020).
NCT04279899 {published data only}
-
- NCT04279899. The investigation of the neonates with or with risk of COVID-19. clinicaltrials.gov/ct2/show/NCT04279899 (first received 27 April 2020).
NCT04285801 {published data only}
-
- NCT04285801. Critically ill patients with COVID-19 in Hong Kong: a multicentre observational cohort study. clinicaltrials.gov/ct2/show/NCT04285801 (first received 27 April 2020).
NCT04292327 {published data only}
-
- NCT04292327. Clinical progressive characteristics and treatment effects of 2019-novel coronavirus. clinicaltrials.gov/ct2/show/NCT04292327 (first received 27 April 2020).
NCT04292964 {published data only}
-
- NCT04292964. Prognostic factors of patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04292964 (first received 27 April 2020).
NCT04315870 {published data only}
-
- NCT04315870. Clinical characteristics of coronavirus disease 2019 (COVID-19) in pregnancy: the Italian Registry on coronavirus in pregnancy. clinicaltrials.gov/ct2/show/NCT04315870 (first received 20 March 2020).
Additional references
Bossuyt 2015
Griffith 2020
Macaskill 2013
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
McInnes 2020
Moher 2009
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutjes 2006
SAS 2015 [Computer program]
-
- SAS Institute SAS. Version 9.4. Cary, NC, USA: SAS Institute, 2015.Available at www.sas.com.
STATA [Computer program]
-
- STATA. Version 16. College Station, TX, USA: StataCorp, 2019.Available at www.stata.com.
Takwoingi 2017
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. - PubMed
References to other published versions of this review
Deeks 2020
-
- Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, Spijker R, et al. Diagnosis of SARS-CoV-2 infection and COVID-19: accuracy of signs and symptoms; molecular, antigen, and antibody tests; and routine laboratory markers. Cochrane Database of Systematic Reviews 2020, Issue 4. [DOI: 10.1002/14651858.CD013596] - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

