Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion
- PMID: 32633861
- PMCID: PMC7388176
- DOI: 10.1002/14651858.CD009510.pub3
Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion
Abstract
Background: Branch retinal vein occlusion (BRVO) is one of the most commonly occurring retinal vascular abnormalities. The most common cause of visual loss in people with BRVO is macular oedema (MO). Grid or focal laser photocoagulation has been shown to reduce the risk of visual loss. Limitations to this treatment exist, however, and newer modalities may have equal or improved efficacy. Antiangiogenic therapy with anti-vascular endothelial growth factor (anti-VEGF) has recently been used successfully to treat MO resulting from a variety of causes.
Objectives: To investigate the efficacy and gather evidence from randomised controlled trials (RCTs) on the potential harms of anti-vascular endothelial growth factor (VEGF) agents for the treatment of macular oedema (MO) secondary to branch retinal vein occlusion (BRVO).
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2019, Issue 6); MEDLINE Ovid; Embase Ovid; the ISRCTN registry; ClinicalTrials.gov; and the WHO ICTRP. The date of the last search was 12 June 2019.
Selection criteria: We included randomised controlled trials (RCTs) investigating BRVO. Eligible trials had to have at least six months' follow-up where anti-VEGF treatment was compared with another treatment, no treatment, or placebo. We excluded trials where combination treatments (anti-VEGF plus other treatments) were used; and trials that investigated the dose and duration of treatment without a comparison group (other treatment/no treatment/sham).
Data collection and analysis: Two review authors independently extracted the data using standard methodological procedures expected by Cochrane. The primary outcome was the proportion of participants with an improvement from baseline in best-corrected visual acuity of greater than or equal to 15 letters (3 lines) on the Early Treatment in Diabetic Retinopathy Study (ETDRS) Chart at six months and 12 months of follow-up. The secondary outcomes were the proportion of participants who lost greater than or equal to 15 ETDRS letters (3 lines) and the mean visual acuity (VA) change at six and 12 months, as well as the change in central retinal thickness (CRT) on optical coherence tomography from baseline at six and 12 months. We also collected data on adverse events and quality of life (QoL).
Main results: We found eight RCTs of 1631 participants that met the inclusion criteria after independent and duplicate review of the search results. These studies took place in Europe, North America, Eastern Mediterranean region and East Asia. Included participants were adults aged 18 or over with VA of 20/40 or worse. Studies varied by duration of disease but permitted previously treated eyes as long as there was sufficient treatment-free interval. All anti-VEGF agents (bevacizumab, ranibizumab and aflibercept) and steroids (triamcinolone and dexamethasone) were included. Overall, we judged the studies to be at moderate or unclear risk of bias. Four of the eight studies did not mask participants or outcome assessors, or both. One trial compared anti-VEGF to sham. At six months, eyes receiving anti-VEGF were significantly more likely to have a gain of 15 or more ETDRS letters (risk ratio (RR) 1.72, 95% confidence interval (CI) 1.19 to 2.49; 283 participants; moderate-certainty evidence). Mean VA was better in the anti-VEGF group at six months compared with control (mean difference (MD) 7.50 letters, 95% CI 5.29 to 9.71; 282 participants; moderate-certainty evidence). Anti-VEGF also proved more effective at reducing CRT at six months (MD -57.50 microns, 95% CI -108.63 to -6.37; 281 participants; lower CRT is better; moderate-certainty evidence). There was only very low-certainty evidence on adverse effects. There were no reports of endophthalmitis. Mean change in QoL (measured using the National Eye Institute Visual Functioning Questionnaire VFQ-25) was better in people treated with anti-VEGF compared with people treated with sham (MD 7.6 higher score, 95% CI 4.3 to 10.9; 281 participants; moderate-certainty evidence). Three RCTs compared anti-VEGF with macular laser (total participants = 473). The proportion of eyes gaining 15 or more letters was greater in the anti-VEGF group at six months (RR 2.09, 95% CI 1.44 to 3.05; 2 studies, 201 participants; moderate-certainty evidence). Mean VA in the anti-VEGF groups was better than the laser groups at six months (MD 9.63 letters, 95% CI 7.23 to 12.03; 3 studies, 473 participants; moderate-certainty evidence). There was a greater reduction in CRT in the anti-VEGF group compared with the laser group at six months (MD -147.47 microns, 95% CI -200.19 to -94.75; 2 studies, 201 participants; moderate-certainty evidence). There was only very low-certainty evidence on adverse events. There were no reports of endophthalmitis. QoL outcomes were not reported. Four studies compared anti-VEGF with intravitreal steroid (875 participants). The proportion of eyes gaining 15 or more ETDRS letters was greater in the anti-VEGF group at six months (RR 1.67, 95% CI 1.33 to 2.10; 2 studies, 330 participants; high-certainty evidence) and 12 months (RR 1.76, 95% CI 1.36 to 2.28; 1 study, 307 participants; high-certainty evidence). Mean VA was better in the anti-VEGF group at six months (MD 8.22 letters, 95% CI 5.69 to 10.76; 2 studies, 330 participants; high-certainty evidence) and 12 months (MD 9.15 letters, 95% CI 6.32 to 11.97; 2 studies, 343 participants; high-certainty evidence). Mean CRT also showed a greater reduction in the anti-VEGF arm at 12 months compared with intravitreal steroid (MD -26.92 microns, 95% CI -65.88 to 12.04; 2 studies, 343 participants; moderate-certainty evidence). People receiving anti-VEGF showed a greater improvement in QoL at 12 months compared to those receiving steroid (MD 3.10, 95% CI 0.22 to 5.98; 1 study, 307 participants; moderate-certainty evidence). Moderate-certainty evidence suggested increased risk of cataract and raised IOP with steroids. There was only very low-certainty evidence on APTC events. No cases of endophthalmitis were observed.
Authors' conclusions: The available RCT evidence suggests that treatment of MO secondary to BRVO with anti-VEGF improves visual and anatomical outcomes at six and 12 months.
Trial registration: ClinicalTrials.gov NCT01396057 NCT01521559 NCT03108352.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
No authors have any financial or proprietary interest in any product mentioned.
Figures
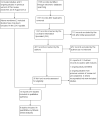




























Update of
-
Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion.Cochrane Database Syst Rev. 2013 Jan 31;(1):CD009510. doi: 10.1002/14651858.CD009510.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Jul 7;7:CD009510. doi: 10.1002/14651858.CD009510.pub3. PMID: 23440840 Updated.
Similar articles
-
Anti-vascular endothelial growth factor for macular oedema secondary to branch retinal vein occlusion.Cochrane Database Syst Rev. 2013 Jan 31;(1):CD009510. doi: 10.1002/14651858.CD009510.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Jul 7;7:CD009510. doi: 10.1002/14651858.CD009510.pub3. PMID: 23440840 Updated.
-
Macular grid laser photocoagulation for branch retinal vein occlusion.Cochrane Database Syst Rev. 2015 May 11;2015(5):CD008732. doi: 10.1002/14651858.CD008732.pub2. Cochrane Database Syst Rev. 2015. PMID: 25961835 Free PMC article.
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2018 Oct 16;10(10):CD007419. doi: 10.1002/14651858.CD007419.pub6. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Jun 27;2023(6):CD007419. doi: 10.1002/14651858.CD007419.pub7. PMID: 30325017 Free PMC article. Updated.
-
Anti-vascular endothelial growth factor combined with intravitreal steroids for diabetic macular oedema.Cochrane Database Syst Rev. 2018 Apr 18;4(4):CD011599. doi: 10.1002/14651858.CD011599.pub2. Cochrane Database Syst Rev. 2018. PMID: 29669176 Free PMC article.
-
Anti-vascular endothelial growth factor for diabetic macular oedema: a network meta-analysis.Cochrane Database Syst Rev. 2017 Jun 22;6(6):CD007419. doi: 10.1002/14651858.CD007419.pub5. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Oct 16;10:CD007419. doi: 10.1002/14651858.CD007419.pub6. PMID: 28639415 Free PMC article. Updated.
Cited by
-
Real-world treatment intensities and pathways of macular edema following retinal vein occlusion in Korea from Common Data Model in ophthalmology.Sci Rep. 2022 Jun 17;12(1):10162. doi: 10.1038/s41598-022-14386-5. Sci Rep. 2022. PMID: 35715561 Free PMC article.
-
Wide-field swept-source OCT angiography of the periarterial capillary-free zone before and after anti-VEGF therapy for branch retinal vein occlusion.Eye Vis (Lond). 2022 Jul 2;9(1):25. doi: 10.1186/s40662-022-00297-z. Eye Vis (Lond). 2022. PMID: 35778771 Free PMC article.
-
Efficacy and safety of a new ranibizumab biosimilar CKD-701 using a pro re nata treatment regimen in neovascular age-related macular degeneration: A phase 3 randomized clinical trial.PLoS One. 2022 Nov 14;17(11):e0275611. doi: 10.1371/journal.pone.0275611. eCollection 2022. PLoS One. 2022. PMID: 36374913 Free PMC article. Clinical Trial.
-
Pharmacokinetic study of Tangwang Mingmu granule for the management of diabetic retinopathy based on network pharmacology.Pharm Biol. 2021 Dec;59(1):1334-1350. doi: 10.1080/13880209.2021.1979051. Pharm Biol. 2021. PMID: 34590544 Free PMC article.
-
Hemiretinal vein occlusion 12-month outcomes are unique with vascular endothelial growth factor inhibitors: data from the Fight Retinal Blindness! Registry.Br J Ophthalmol. 2023 Jun;107(6):842-848. doi: 10.1136/bjophthalmol-2021-320482. Epub 2022 Jan 25. Br J Ophthalmol. 2023. PMID: 35078771 Free PMC article.
References
References to studies included in this review
Bandello 2018 {published and unpublished data}
-
- EU-CTR 2010-023900-29. A 12-month, multicentre, randomised, parallel group study to compare the efficacy and safety of OZURDEX® versus Lucentis® in patients with branch retinal vein occlusion. www.clinicaltrialsregister.eu/ctr-search/trial/2010-023900-29/results (first received 28 August 2011).
-
- NCT01427751. Safety and efficacy study of Ozurdex® compared to Lucentis® in patients with branch retinal vein occlusion. clinicaltrials.gov/ct2/show/NCT01427751 (first received 2 September 2011).
BLOSSOM {unpublished data only}
-
- NCT01976338. Ranibizumab intravitreal injections versus sham control in patients with branch retinal vein occlusion (BRVO) (Blossom). ClinicalTrials.gov/show/NCT01976338 (first received 5 November 2013).
-
- Wei W. To assess the efficacy and safety of individualized intravitreal ranibizumab 0.5 mg in Asian (primarily Chinese) patients with visual impairment due to macular edema secondary to branch retinal vein occlusion (BRVO). In: Annual Meeting of the Association for Research in Vision and Ophthalmology. 2017.
BRIGHTER {published data only}
-
- Tadayoni R, Waldstein SM, Boscia F, Gerding H, Gekkieva M, Barnes E, et al. Sustained benefits of ranibizumab with or without laser in branch retinal vein occlusion: 24-month results of the BRIGHTER study. Ophthalmology 2017;124(12):1778-87. - PubMed
-
- Tadayoni R, Waldstein SM, Boscia F, Gerding H, Pearce I, Priglinger S, et al. Individualized stabilization criteria-driven ranibizumab versus laser in branch retinal vein occlusion: six-month results of BRIGHTER. Ophthalmology 2016;123(6):1332-44. - PubMed
COMRADE‐B {published data only}
-
- Hattenbach LO, Feltgen N, Bertelmann T, Schmitz-Valckenberg S, Berk H, Eter N, et al. Head-to-head comparison of ranibizumab PRN versus single-dose dexamethasone for branch retinal vein occlusion (COMRADE-B). Acta Ophthalmologica 2018;96(1):e10-8. - PubMed
Higashiyama 2013 {published data only}
-
- Higashiyama T, Sawada O, Kakinoki M, Sawada T, Kawamura H, Ohji M. Prospective comparisons of intravitreal injections of triamcinolone acetonide and bevacizumab for macular oedema due to branch retinal vein occlusion. Acta Ophthalmologica 2013;91(4):318-24. - PubMed
RABAMES {published data only}
-
- Pielen A, Mirshahi A, Feltgen N, Lorenz K, Korb C, Junker B, et al. Ranibizumab for branch retinal vein occlusion associated macular edema study (RABAMES): six-month results of a prospective randomized clinical trial. Acta Ophthalmologica 2015;93(1):e29-37. - PubMed
Ramezani 2012 {published data only}
-
- Ramezani A, Esfandiari H, Entezari M, Moradian S, Soheilian M, Dehsarvi B, et al. Three intravitreal bevacizumab versus two intravitreal triamcinolone injections in recent-onset branch retinal vein occlusion. Graefe's Archive for Clinical and Experimental Ophthalmology 2012;250(8):1149-60. - PubMed
VIBRANT {published data only}
-
- Campochiaro PA, Clark WL, Boyer DS, Heier JS, Brown DM, Vitti R, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: the 24-week results of the VIBRANT study. Ophthalmology 2015;122(3):538-44. - PubMed
-
- Clark WL, Boyer DS, Heier JS, Brown DM, Haller JA, Vitti R, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology 2016;123(2):330-6. - PubMed
References to studies excluded from this review
BRAVO 2010 {published data only}
-
- Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology 2011;118(8):1594-602. - PubMed
-
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117(6):1102-12. - PubMed
Campochiaro 2008 {published data only}
-
- Campochiaro PA, Hafiz G, Shah SM, Nguyen QD, Ying H, Do DV, et al. Ranibizumab for macular edema due to retinal vein occlusions: implication of VEGF as a critical stimulator. Molecular Therapy 2008;16(4):791-9. - PubMed
Campochiaro 2010a {published data only}
-
- Campochiaro PA, Hafiz G, Channa R, Shah SM, Nguyen QD, Ying H, et al. Antagonism of vascular endothelial growth factor for macular edema caused by retinal vein occlusions: two-year outcomes. Ophthalmology 2010;117(12):2387-94. - PubMed
Chiquet 2015 {published data only}
-
- Chiquet C, Dupuy C, Bron AM, Aptel F, Straub M, Isaico R, et al. Intravitreal dexamethasone implant versus anti-VEGF injection for treatment-naive patients with retinal vein occlusion and macular edema: a 12-month follow-up study. Graefe's Archive for Clinical and Experimental Ophthalmology 2015;253(12):2095-102. - PubMed
Chiquet 2016 {published data only}
-
- Chiquet C, Bron AM, Straub M, Dupuy C, Isaico R, Aptel F, et al. Retinal vein occlusions: therapeutic switch in macular oedema treatment with a 12-month follow-up. Ophthalmic Research 2016;55(3):152-8. - PubMed
COMRADE Extension 2018 {published data only}
-
- Feltgen N, Hattenbach LO, Bertelmann T, Callizo J, Rehak M, Wolf A, et al. Comparison of ranibizumab versus dexamethasone for macular oedema following retinal vein occlusion: 1-year results of the COMRADE extension study. Acta Ophthalmologica 2018;96(8):e933-e941. - PubMed
CRAVE 2015 {published data only}
-
- Rajagopal R, Shah GK, Blinder KJ, Altaweel M, Eliott D, Wee R, et al. Bevacizumab versus ranibizumab in the treatment of macular edema due to retinal vein occlusion: 6-month results of the CRAVE study. Ophthalmic Surgery, Lasers & Imaging Retina 2015;46(8):844-50. - PubMed
Donati 2012 {published data only}
-
- Donati S, Barosi P, Bianchi M, Al Oum M, Azzolini C. Combined intravitreal bevacizumab and grid laser photocoagulation for macular edema secondary to branch retinal vein occlusion. European Journal of Ophthalmology 2012;22(4):607-14. - PubMed
Gu 2017 {published data only}
-
- Gu X, Yu X, Song S, Dai H. Intravitreal dexamethasone implant versus intravitreal ranibizumab for the treatment of macular edema secondary to retinal vein occlusion in a Chinese population. Ophthalmic Research 2017;58(1):8-14. - PubMed
Guignier 2013 {published data only}
-
- Guignier B, Subilia-Guignier A, Fournier I, Ballonzoli L, Speeg-Schatz C, Gaucher D. Prospective pilot study: efficacy of intravitreal dexamethasone and bevacizumab injections in the treatment of macular oedema associated with branch retinal vein occlusion. Ophthalmologica 2013;230(1):43-9. - PubMed
Hanhart 2017 {published data only}
-
- Hanhart J, Rozenman Y. Comparison of intravitreal ranibizumab, aflibercept, and dexamethasone implant after bevacizumab failure in macular edema secondary to retinal vascular occlusions. Ophthalmologica 2017;238(1-2):110-8. - PubMed
Kartasasmita 2016 {published data only}
Klimes 2015 {published data only}
-
- Klimes J, Regnier SA, Mahon R, Budek T, Dostal F, Skalicky D, Depta J. Cost effectiveness analysis of ranibizumab compared to aflibercept and laser intervention In treatment of diabetic macular edema (DME) In the Czech Republic. Value in Health 2015;18:A 419.
Leitritz 2013 {published data only}
-
- Leitritz MA, Gelisken F, Ziemssen F, Szurman P, Bartz-Schmidt KU, Jaissle GB. Grid laser photocoagulation for macular oedema due to branch retinal vein occlusion in the age of bevacizumab? Results of a prospective study with crossover design. British Journal of Ophthalmology 2013;97(2):215-9. - PubMed
Liu 2014 {published data only}
-
- Liu B, Yang Y, Liu X, Li W, Mo Z. Clinical therapeutic effects of intravitreal Ranibizumab injection combined laser photocoagulation for macular edema in BRVO. Guoji Yanke Zazhi 2014;14(11):2006-8.
MARVEL {published data only}
-
- Narayanan R, Panchal B, Das T, Chhablani J, Jalali S, Ali MH, et al. A randomised, double-masked, controlled study of the efficacy and safety of intravitreal bevacizumab versus ranibizumab in the treatment of macular oedema due to branch retinal vein occlusion: MARVEL Report No. 1. British Journal of Ophthalmology 2015;99(7):954–9. - PubMed
Moon 2016 {published data only}
Moradian 2011 {published data only}
-
- Moradian S, Faghihi H, Sadeghi B, Piri N, Ahmadieh H, Soheilian M, et al. Intravitreal bevacizumab vs. sham treatment in acute branch retinal vein occlusion with macular edema: results at 3 months (Report 1). Graefe's Archive for Clinical and Experimental Ophthalmology 2011;249(2):193-200. - PubMed
Ramezani 2014 {published data only}
-
- Ramezani A, Esfandiari H, Entezari M, Moradian S, Soheilian M, Dehsarvi B, et al. Three intravitreal bevacizumab versus two intravitreal triamcinolone injections in recent onset central retinal vein occlusion. Acta Ophthalmologica 2014;92(7):e530-9. - PubMed
Regnier 2015 {published data only}
Russo 2009 {published data only}
-
- Russo V, Barone A, Conte E, Prascina F, Stella A, Noci ND. Bevacizumab compared with macular laser grid photocoagulation for cystoid macular edema in branch retinal vein occlusion. Retina 2009;29(4):511-5. - PubMed
SHORE 2014 {published data only}
-
- Campochiaro PA, Wykoff CC, Singer M, Johnson R, Marcus D, Yau L, et al. Monthly versus as-needed ranibizumab injections in patients with retinal vein occlusion: the SHORE study. Ophthalmology 2014;121(12):2432-42. - PubMed
Tan 2014 {published data only}
-
- Tan MH, McAllister IL, Gillies ME, Verma N, Banerjee, Smithies LA, et al. Randomized controlled trial of intravitreal ranibizumab versus standard grid laser for macular edema following branch retinal vein occlusion. American Journal of Ophthalmology 2014;157(1):237-47. - PubMed
Tomomatsu 2016 {published data only}
-
- Tomomatsu Y, Tomomatsu T, Takamura Y, Gozawa M, Arimura S, Takihara Y, et al. Comparative study of combined bevacizumab/targeted photocoagulation vs bevacizumab alone for macular oedema in ischaemic branch retinal vein occlusions. Acta Ophthalmologica 2016;94(3):e225-30. - PubMed
Wroblewski 2010 {published data only}
-
- Wroblewski JJ, Wells JA 3rd, Gonzales CR. Pegaptanib sodium for macular edema secondary to branch retinal vein occlusion. American Journal of Ophthalmology 2010;149(1):147-54. - PubMed
Zhang 2014 {published data only}
-
- Zhang XY, Li YB, Zhou LW, Wang XB, Luo XD. Observation on intravitreal injection of ranibizumab combined with laser photocoagulation for the treatment of macular edema in BRVO. International Eye Science 2014;14:747-9.
References to ongoing studies
NCT01189526 {unpublished data only}
-
- NCT01189526. Comparison of intravitreal ranibizumab and macular laser photocoagulation for ME following branch retinal vein occlusion (BRVO). clinicaltrials.gov/ct2/show/NCT01189526 (first received 26 August 2010).
NCT01795209 {unpublished data only}
-
- NCT01795209. Ranibizumab for macular edema secondary to branch retinal vein occlusion in patients with fair vision (RVOFV). clinicaltrials.gov/ct2/show/NCT01795209 (first received 20 February 2013).
NCT03108352 {unpublished data only}
-
- NCT03108352. Conbercept ophthalmic injection for patients with macular edema caused by branch retinal vein occlusion. clinicaltrials.gov/ct2/show/NCT03108352 (first received 11 April 2017).
Additional references
Abegg 2008
Ahmadi 2009
-
- Ahmadi AA, Chuo JY, Banashkevich A, Ma PE, Maberley DA. The effects of intravitreal bevacizumab on patients with macular edema secondary to branch retinal vein occlusion. Canadian Journal of Ophthalmology 2009;44(2):154-9. - PubMed
Aiello 1995a
-
- Aiello LP, Pierce EA, Foley ED, Takagi H, Chen H, Riddle L, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proceedings of the National Academy of Sciences of the United States of America 1995;92(23):10457-61. - PMC - PubMed
Aiello 1995b
-
- Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Archives of Ophthalmology 1995;113(12):1538-44. - PubMed
Awdeh 2010
-
- Awdeh RM, Elsing SH, Deramo VA, Stinnett S, Lee PP, Fekrat S. Vision-related quality of life in persons with unilateral branch retinal vein occlusion using the 25-item National Eye Institute Visual Function Questionnaire. British Journal of Ophthalmology 2010;94(3):319-23. - PubMed
Boyd 2002
-
- Boyd SR, Zachary I, Chakravarthy U, Allen GJ, Wisdom GB, Cree IA, et al. Correlation of increased vascular endothelial growth factor with neovascularization and permeability in ischemic central vein occlusion. Archives of Ophthalmology 2002;120(12):1644-50. - PubMed
BVOS Group 1984
-
- The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. American Journal of Ophthalmology 1984;98(3):271-82. - PubMed
Byeon 2007
-
- Byeon SH, Kwon YA, Oh HS, Kim M, Kwon OW. Short-term results of intravitreal bevacizumab for macular edema with retinal vein obstruction and diabetic macular edema. Journal of Ocular Pharmacology and Therapeutics 2007;23(4):387-94. - PubMed
Byun 2010
-
- Byun YJ, Roh MI, Lee SC, Koh HJ. Intravitreal triamcinolone acetonide versus bevacizumab therapy for macular edema associated with branch retinal vein occlusion. Graefe's Archive for Clinical and Experimental Ophthalmology 2010;248(7):963-71. - PubMed
Campochiaro 2010b
-
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117(6):1102-12. - PubMed
Cekic 2010
-
- Cekic O, Cakir M, Yazici AT, Alagoz N, Bozkurt E, Faruk Yilmaz O. A comparison of three different intravitreal treatment modalities of macular edema due to branch retinal vein occlusion. Current Eye Research 2010;35(10):925-9. - PubMed
Chen 2010
-
- Chen CH, Chen YH, Wu PC, Chen YJ, Lee JJ, Liu YC, et al. Treatment of branch retinal vein occlusion induced macular edema in treatment-naive cases with a single intravitreal triamcinolone or bevacizumab injection. Chang Gung Medical Journal 2010;33(4):424-35. - PubMed
Cheng 2009
-
- Cheng KC, Wu WC, Chen KJ. Intravitreal triamcinolone acetonide vs bevacizumab for treatment of macular oedema secondary to branch retinal vein occlusion. Eye 2009;23(11):2023-33. - PubMed
Chung 2008
-
- Chung EJ, Hong YT, Lee SC, Kwon OW, Koh HJ. Prognostic factors for visual outcome after intravitreal bevacizumab for macular edema due to branch retinal vein occlusion. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(9):1241-7. - PubMed
Deeks 2017
-
- Deeks JJ, Higgins JP, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Dodson 1982
Dodson 1992
-
- Dodson PM, Kritzinger EE, Clough CG. Diabetes mellitus and retinal vein occlusion in patients of Asian, West Indian and white European origin. Eye 1992;6(1):66-8. - PubMed
EDCCS Group 1993
-
- Anonymous. Risk factors for branch retinal vein occlusion. The Eye Disease Case-Control Study Group. American Journal of Ophthalmology 1993;116(3):286-96. - PubMed
Ehlers 2011
-
- Ehlers JP, Fekrat S. Retinal vein occlusion: beyond the acute event. Survey of Ophthalmology 2011;56(4):281-99. - PubMed
Ferrara 2006
-
- Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 2006;26(8):859-70. - PubMed
Figueroa 2010
-
- Figueroa MS, Contreras I, Noval S, Arruabarrena C. Results of bevacizumab as the primary treatment for retinal vein occlusions. British Journal of Ophthalmology 2010;94(8):1052-6. - PubMed
Frangieh 1982
-
- Frangieh GT, Green WR, Barraquer-Somers E, Finkelstein D. Histopathologic study of nine branch retinal vein occlusions. Archives of Ophthalmology 1982;100(7):1132-40. - PubMed
Funk 2009
-
- Funk M, Kriechbaum K, Prager F, Benesch T, Georgopoulos M, Zlabinger GJ, et al. Intraocular concentrations of growth factors and cytokines in retinal vein occlusion and the effect of therapy with bevacizumab. Investigative Ophthalmology and Visual Science 2009;50(3):1025-32. - PubMed
Garnock‐Jones 2011
-
- Garnock-Jones KP. Ranibizumab: in macular oedema following retinal vein occlusion. Drugs 2011;71(4):455-63. - PubMed
Glacet‐Bernard 1996
-
- Glacet-Bernard A, Coscas G, Chabanel A, Zourdani A, Lelong F, Samama MM. Prognostic factors for retinal vein occlusion: prospective study of 175 cases. Ophthalmology 1996;103(4):551-60. - PubMed
Glanville 2006
GRADE 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors The GRADE Working Group. GRADE Handbook for grading quality of evidence and strength of recommendations. gdt.gradepro.org/app/handbook/handbook.html (initially accessed 2013).
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed prior to 11 July 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).Available at gradepro.org.
Gragoudas 2004
-
- Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. New England Journal of Medicine 2004;351(27):2805-16. - PubMed
Gregori 2009
-
- Gregori NZ, Rattan GH, Rosenfeld PJ, Puliafito CA, Feuer W, Flynn HW Jr, et al. Safety and efficacy of intravitreal bevacizumab (avastin) for the management of branch and hemiretinal vein occlusion. Retina 2009;29(7):913-25. - PubMed
Gunduz 2008
-
- Gunduz K, Bakri SJ. Intravitreal bevacizumab for macular oedema secondary to branch retinal vein occlusion. Eye 2008;22(9):1168-71. - PubMed
Haller 2010
-
- Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology 2010;117(6):1134-46. - PubMed
Hara 2010
-
- Hara S, Sakuraba T, Kataoka H, Yanagihashi S, Saitou K, Noda Y. Effect of repeated intravitreal bevacizumab injections for secondary macular edema of branch retinal vein occlusion. Nihon Ganka Gakkai Zasshi 2010;114(12):1013-8. - PubMed
Higgins 2019
-
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Hoeh 2009
-
- Hoeh AE, Ach T, Schaal KB, Scheuerle AF, Dithmar S. Long-term follow-up of OCT-guided bevacizumab treatment of macular edema due to retinal vein occlusion. Graefe's Archive for Clinical and Experimental Ophthalmology 2009;247(12):1635-41. - PubMed
Hou 2009
-
- Hou J, Tao Y, Jiang YR, Li XX, Gao L. Intravitreal bevacizumab versus triamcinolone acetonide for macular edema due to branch retinal vein occlusion: a matched study. Chinese Medical Journal 2009;122(22):2695-9. - PubMed
Hung 2010
-
- Hung KH, Lee SM, Lee SY, Lee FL, Yang CS. Intravitreal bevacizumab (avastin) in the treatment of macular edema associated with perfused retinal vein occlusion. Journal of Ocular Pharmacology and Therapeutics 2010;26(1):85-90. - PubMed
Jaissle 2009
-
- Jaissle GB, Leitritz M, Gelisken F, Ziemssen F, Bartz-Schmidt KU, Szurman P. One-year results after intravitreal bevacizumab therapy for macular edema secondary to branch retinal vein occlusion. Graefe's Archive for Clinical and Experimental Ophthalmology 2009;247(1):27-33. - PubMed
Jaissle 2011
-
- Jaissle GB, Szurman P, Feltgen N, Spitzer B, Pielen A, Rehak M, et al. Predictive factors for functional improvement after intravitreal bevacizumab therapy for macular edema due to branch retinal vein occlusion. Graefe's Archive for Clinical and Experimental Ophthalmology 2011;249(2):183-92. - PMC - PubMed
Klein 2000
Klein 2008
-
- Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Archives of Ophthalmology 2008;126(4):513-8. - PubMed
Kondo 2009
-
- Kondo M, Kondo N, Ito Y, Kachi S, Kikuchi M, Yasuma TR, et al. Intravitreal injection of bevacizumab for macular edema secondary to branch retinal vein occlusion:results after 12 months and multiple regression analysis. Retina 2009;29(9):1242-8. - PubMed
Kourlas 2007
-
- Kourlas H, Abrams P. Ranibizumab for the treatment of neovascular age-related macular degeneration: a review. Clinical Therapeutics 2007;29(9):1850-61. - PubMed
Kreutzer 2008
-
- Kreutzer TC, Alge CS, Wolf AH, Kook D, Burger J, Strauss R, et al. Intravitreal bevacizumab for the treatment of macular oedema secondary to branch retinal vein occlusion. British Journal of Ophthalmology 2008;92(3):351-5. - PubMed
Kriechbaum 2008
-
- Kriechbaum K, Michels S, Prager F, Georgopoulos M, Funk M, Geitzenauer W, et al. Intravitreal Avastin for macular oedema secondary to retinal vein occlusion: a prospective study. British Journal of Ophthalmology 2008;92(4):518-22. - PubMed
McIntosh 2007
-
- McIntosh RL, Mohamed Q, Saw SM, Wong TY. Interventions for branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology 2007;114(5):835-54. - PubMed
Meander 2014
Mehany 2010
Mitchell 1996
-
- Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Archives of Ophthalmology 1996;114(10):1243-7. - PubMed
Mitchell 2011
-
- Mitchell P. A systematic review of the efficacy and safety outcomes of anti-VEGF agents used for treating neovascular age-related macular degeneration: comparison of ranibizumab and bevacizumab. Current Medical Research and Opinion 2011;27(7):1465-75. - PubMed
Noma 2006
-
- Noma H, Minamoto A, Funatsu H, Tsukamoto H, Nakano K, Yamashita H, et al. Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefe's Archive for Clinical and Experimental Ophthalmology 2006;244(3):309-15. - PubMed
Ozaki 1997
-
- Ozaki H, Hayashi H, Vinores SA, Moromizato Y, Campochiaro PA, Oshima K. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Experimental Eye Research 1997;64(4):505-17. - PubMed
Pai 2007
-
- Pai SA, Shetty R, Vijayan PB, Venkatasubramaniam G, Yadav NK, Shetty BK, et al. Clinical, anatomic, and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. American Journal of Ophthalmology 2007;143(4):601-6. - PubMed
Pece 2011
-
- Pece A, Isola V, Piermarocchi S, Calori G. Efficacy and safety of anti-vascular endothelial growth factor (VEGF) therapy with intravitreal ranibizumab (Lucentis) for naive retinal vein occlusion: 1-year follow-up. British Journal of Ophthalmology 2011;95(1):56-68. - PubMed
Prager 2009
-
- Prager F, Michels S, Kriechbaum K, Georgopoulos M, Funk M, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. British Journal of Ophthalmology 2009;93(4):452-6. - PubMed
Presta 1997
-
- Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Research 1997;57(20):4593-9. - PubMed
Qian 2017
-
- Qian T, Zhao M, Xu X. Comparison between anti-VEGF therapy and corticosteroid or laser therapy for macular oedema secondary to retinal vein occlusion: a meta-analysis. Journal of Clinical Pharmacy and Therapeutics 2017;42(5):519-29. - PubMed
Rabena 2007
-
- Rabena MD, Pieramici DJ, Castellarin AA, Nasir MA, Avery RL. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina 2007;27(4):419-25. - PubMed
Rehak 2008
Rensch 2009
-
- Rensch F, Jonas JB, Spandau UH. Early intravitreal bevacizumab for non-ischaemic branch retinal vein occlusion. Ophthalmologica 2009;223(2):124-7. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogers 2010a
-
- Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW, et al. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology 2010;117(6):1094-101. - PubMed
Rogers 2010b
Rosenfeld 2006
-
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. New England Journal of Medicine 2006;355(14):1419-31. - PubMed
Rouvas 2010
-
- Rouvas A, Petrou P, Ntouraki A, Douvali M, Ladas I, Vergados I. Intravitreal ranibizumab (Lucentis) for branch retinal vein occlusion-induced macular edema: nine-month results of a prospective study. Retina 2010;30(6):893-902. - PubMed
Schaal 2007
-
- Schaal KB, Hoh AE, Scheuerle A, Schutt F, Dithmar S. Bevacizumab for the treatment of macular edema secondary to retinal vein occlusion. Ophthalmologe 2007;104(4):285-9. - PubMed
Scott 2009
-
- Scott IU, Ip MS, VanVeldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Archives of Ophthalmology 2009;127(9):1115-28. - PMC - PubMed
Shilling 1976
Stahl 2007
-
- Stahl A, Agostini H, Hansen LL, Feltgen N. Bevacizumab in retinal vein occlusion-results of a prospective case series. Graefe's Archive for Clinical and Experimental Ophthalmology 2007;245(10):1429-36. - PubMed
Wong 2005
-
- Wong TY, Larsen EK, Klein R, Mitchell P, Couper DJ, Klein BE, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities & Cardiovascular Health studies. Ophthalmology 2005;112(4):540-7. - PubMed
Xu 2007
-
- Xu L, Liu WW, Wang YX, Yang H, Jonas JB. Retinal vein occlusions and mortality: the Beijing Eye Study. American Journal of Ophthalmology 2007;144(6):972-3. - PubMed
Yasuda 2010
-
- Yasuda M, Kiyohara Y, Arakawa S, Hata Y, Yonemoto K, Doi Y, et al. Prevalence and systemic risk factors for retinal vein occlusion in a general Japanese population: the Hisayama study. Investigative Ophthalmology and Visual Science 2010;51(6):3205-9. - PubMed
References to other published versions of this review
Mitry 2011
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

