Cell-free DNA profiling in retinoblastoma patients with advanced intraocular disease: An MSKCC experience
- PMID: 32633890
- PMCID: PMC7476838
- DOI: 10.1002/cam4.3144
Cell-free DNA profiling in retinoblastoma patients with advanced intraocular disease: An MSKCC experience
Abstract
Purpose: The enucleation rate for retinoblastoma has dropped from over 95% to under 10% in the past 10 years as a result of improvements in therapy. This reduces access to tumor tissue for molecular profiling, especially in unilateral retinoblastoma, and hinders the confirmation of somatic RB1 mutations necessary for genetic counseling. Plasma cell-free DNA (cfDNA) has provided a platform for noninvasive molecular profiling in cancer, but its applicability in low tumor burden retinoblastoma has not been shown. We analyzed cfDNA collected from 10 patients with available tumor tissue to determine whether sufficient tumorderived cfDNA is shed in plasma from retinoblastoma tumors to enable noninvasive RB1 mutation detection.
Methods: Tumor tissue was collected from eye enucleations in 10 patients diagnosed with advanced intra-ocular unilateral retinoblastoma, three of which went on to develop metastatic disease. Tumor RB1 mutation status was determined using an FDA-cleared tumor sequencing assay, MSK-IMPACT. Plasma samples were collected before eye enucleation and analyzed with a customized panel targeting all exons of RB1.
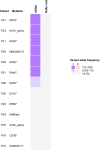
Results: Tumor-guided genotyping detected 10 of the 13 expected somatic RB1 mutations in plasma cfDNA in 8 of 10 patients (average variant allele frequency 3.78%). Without referring to RB1 status in the tumor, de novo mutation calling identified 7 of the 13 expected RB1 mutations (in 6 of 10 patients) with high confidence.
Conclusion: Plasma cfDNA can detect somatic RB1 mutations in patients with unilateral retinoblastoma. Since intraocular biopsies are avoided in these patients because of concern about spreading tumor, cfDNA can potentially offer a noninvasive platform to guide clinical decisions about treatment, follow-up schemes, and risk of metastasis.
Keywords: RB1 mutation; liquid biopsy; molecular profiling in retinoblastoma; plasma cell-free DNA; retinoblastoma.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
PK, JY, CMS, DS, XJ, JE, KH, AV, JHF, NS, and DHA have nothing to disclose. MFB, DWYT, JP, MH, and FM are co‐inventors on a provisional patent application for systems and methods for detecting cancer via cfDNA screening (PCT/US2019/027487). DT and FM are co‐inventors on a provisional patent application for systems and methods for distinguishing pathological mutations from clonal hematopoietic mutations in plasma cell‐free DNA by fragment size analysis. MFB has received consulting fees from Roche and grant support from Illumina and Grail. DT has received research support from ThermoFisher Scientific, EPIC Sciences, speaking honoraria and travel support from Nanodigmbio, Cowen, BoA Merrill Lynch. IJD reports non‐financial support from Apexigen, personal fees from Bayer, grants from Bristol‐Myers Squibb, personal fees from Celgene, grants from Genentech, grants from Novartis, outside the submitted work.
Figures


References
-
- Ries LAGSM, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR (eds). Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute; 1999.
-
- Dimaras H, Kimani K, Dimba EAO, et al. Retinoblastoma. Lancet (London, England). 2012;379(9824):1436‐1446. - PubMed
-
- Broaddus E, Topham A, Singh AD. Incidence of retinoblastoma in the USA: 1975–2004. The British journal of ophthalmology. 2009;93(1):21‐23. - PubMed
-
- Brichard B, Heusterspreute M, De Potter P, et al. Unilateral retinoblastoma, lack of familial history and older age does not exclude germline RB1 gene mutation. Eur J cancer (Oxford, England : 1990). 2006;42(1):65‐72. - PubMed
-
- Mendoza PR, Grossniklaus HE. The Biology of Retinoblastoma. Progress in molecular biology and translational science. 2015;134:503‐516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

