SARS-CoV-2 viral load and antibody responses: the case for convalescent plasma therapy
- PMID: 32634126
- PMCID: PMC7524464
- DOI: 10.1172/JCI139760
SARS-CoV-2 viral load and antibody responses: the case for convalescent plasma therapy
Abstract
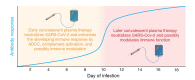
Most patients with COVID-19 lack antibody to SARS-CoV-2 in the first 10 days of illness while the virus drives disease pathogenesis. SARS-CoV-2 antibody deficiency in the setting of a tissue viral burden suggests that using an antibody as a therapeutic agent would augment the antiviral immune response. In this issue of the JCI, Wang and collaborators describe the kinetics of viral load and the antibody responses of 23 individuals with COVID-19 experiencing mild and severe disease. The researchers found that (a) individuals with mild and severe disease produced neutralizing IgG to SARS-CoV-2 10 days after disease onset, (b) SARS-CoV-2 persisted longer in those with severe disease, and (c) there was cross-reactivity between antibodies to SARS-CoV-1 and SARS-CoV-2, but only antibodies from patients with COVID-19 neutralized SARS-CoV-2. These observations provide important information on the serological response to SARS-CoV-2 of hospitalized patients with COVID-19 that can inform the use of convalescent plasma therapy.
Conflict of interest statement
Figures
Comment on
-
Kinetics of viral load and antibody response in relation to COVID-19 severity.J Clin Invest. 2020 Oct 1;130(10):5235-5244. doi: 10.1172/JCI138759. J Clin Invest. 2020. PMID: 32634129 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous