Human Cells Grown With or Without Substitutes for Fetal Bovine Serum
- PMID: 32634183
- PMCID: PMC6172986
- DOI: 10.1177/2155179018755140
Human Cells Grown With or Without Substitutes for Fetal Bovine Serum
Abstract
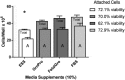
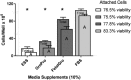
Safety concerns over cell-derived pharmaceutical products being manufactured in supplements of fetal bovine serum (FBS) have ignited pleas to replace FBS. Herein, four newly marketed alternatives to FBS were compared: a xeno-free product called Cell-Ess®, a human platelet lysate marketed as GroPro®, and two mixtures of adult bovine serum varying in their proportions of neonatal growth factors, called Liporo® and FetalGro®. An endothelial cell line (C2BBe1) and a neuronal cell line (SHSY5Y) near confluency in media with 10% FBS were selectively scraped and taken through a 25-day step-wise algorithm to replace FBS, and another human endothelial cell line (HRA-19) was studied to replicate C2BBe1. Cells were stained, counted, and compared for viability, migration, and spheroids. The C2BBe1 and HRA-19 cell lines failed to proliferate in 10% Cell-Ess® but grew in 10% GroPro® or 10% FetalGro® reasonably well compared to reference 10% FBS. With SH-SY5Y, only FetalGro® approached FBS's efficacy. These were all inferior to 11 different branded lots of FBS (positive controls), but five days into switching just amongst the FBS brands, 4 of 11 supported less proliferation than reference FBS in endothelial HRA-19 (p < 0.004). Moreover, neurospheres were enriched in two branded lots of FBS and FetalGro® (each p < 0.004), neurospheres being an unwanted phenotype for any neuronal cell application. Because platelet-derived GroPro® stood out amongst the non-FBS growth supplements to allow proliferation without inducing spheroids, it seems the best (mindful that the cells still grew slower in it compared to FBS). While no perfect replacement was found amongst the alternatives to FBS, the algorithm for switching should be useful in future testing of new alternatives to FBS as the need arises to switch from FBS and expand pharmaceutical products with safety for human use.
Keywords: C2BBe1; SH-SY5Y; fetal bovine serum; neurospheres; xeno-free.
© The Author(s) 2018.
Conflict of interest statement
Declaration of Conflicting Interests: We have no conflicting interests to declare with vendors, agencies, or other interest groups. The sera came to us free of charge on a trial basis, unencumbered by material transfer agreements, restrictions on publication, or mention of intellectual property. Each vendor of FBS was provided the courtesy of a detailed scientific synopsis delivered approximately 2 years prior to the paper being written or submitted.
Figures




References
-
- Van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, Hellebrekers L, Hyllner J, Jonker FH, Prieto P, et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicology In Vitro. 2004;18(1):1–12. - PubMed
-
- Van der Valk J, Bieback K, Buta C, Cochrane B, Dirks WG, Fu J, Hickman JJ, Hohensee C, Kolar R, Liebsch M, et al. Fetal bovine serum (FBS): Past - Present - Future. ALTEX. 2018;35(1):99–118. - PubMed
-
- Davis D, Hirschi SD. Fetal bovine serum: what you should ask your supplier and why. BioProcessing J. 2014;13(1):1–3.
LinkOut - more resources
Full Text Sources

