Laboratory Assay Evaluation Demystified: A Review of Key Factors Influencing Interpretation of Test Results Using Different Assays for SARS-CoV-2 Infection Diagnosis
- PMID: 32634229
- PMCID: PMC7454829
- DOI: 10.1093/labmed/lmaa045
Laboratory Assay Evaluation Demystified: A Review of Key Factors Influencing Interpretation of Test Results Using Different Assays for SARS-CoV-2 Infection Diagnosis
Abstract
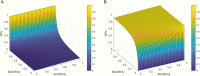
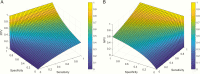
Laboratory tests are an integral part of the diagnosis and management of patients; however, these tests are far from perfect. Their imperfections can be due to patient health condition, specimen collection, and/or technological difficulty with performing the assay and/or interpretation. To be useful clinically, testing requires calculation of positive predictive values (PPVs) and negative predictive values (NPVs). During the current global pandemic of COVID-19 (coronavirus disease 2019), multiple assays with unknown clinical sensitivity and specificity have been rapidly developed to aid in the diagnosis of the disease. Due to a lack of surveillance testing, the prevalence of COVID-19 remains unknown. Hence, using this situation as an clinical example, the goal of this article is to clarify the key factors that influence the PPV and NPV yielded by diagnostic testing, By doing so, we hope to offer health-care providers information that will help them better understand the potential implications of utilizing these test results in clinical patient management.
Keywords: clinical interpretation; negative predictive value; positive predictive value; sensitivity; specificity; test parameters.
© American Society for Clinical Pathology 2020. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
About the Journal.Lab Med. 2020 Sep 1;51(5):443. doi: 10.1093/labmed/lmaa068. Lab Med. 2020. PMID: 32869104 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

