Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura
- PMID: 32634236
- PMCID: PMC7362370
- DOI: 10.1182/bloodadvances.2020001973
Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura
Erratum in
-
Völker LA, Kaufeld J, Miesbach W, et al. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood Adv. 2020;4(13):3085-3092.Blood Adv. 2022 Apr 12;6(7):2434. doi: 10.1182/bloodadvances.2021006915. Blood Adv. 2022. PMID: 35404433 Free PMC article. No abstract available.
Abstract
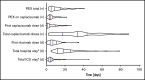
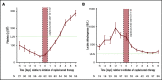
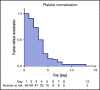
Acquired thrombotic thrombocytopenic purpura (aTTP) is a rare but life-threatening condition. In 2018, the nanobody caplacizumab was approved for the treatment of adults experiencing an acute episode of aTTP, in conjunction with plasma exchange (PEX) and immunosuppression for a minimum of 30 days after stopping daily PEX. We performed a retrospective, observational analysis on the use of caplacizumab in 60 patients from 29 medical centers in Germany during acute disease management. Caplacizumab led to a rapid normalization of the platelet count (median, 3 days; mean 3.78 days). One patient died after late treatment initiation due to aTTP-associated complications. In 2 patients with initial disease presentation and in 4 additional patients with laboratory signs of an exacerbation or relapse after the initial therapy, PEX-free treatment regimens could be established with overall favorable outcome. Caplacizumab is efficacious in the treatment of aTTP independent of timing and ancillary treatment modalities. Based on this real-world experience and published literature, we propose to administer caplacizumab immediately to all patients with an acute episode of aTTP. Treatment decisions regarding the use of PEX should be based on the severity of the clinical presentation and known risk factors. PEX might be dispensable in some patients.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.T.B. reports grants from the German Research Foundation (BR2955/8) during the conduct of the study and personal fees from Alexion, Astellas, Bayer, Sanofi Genzyme, Pfizer, and Vifor (speaker honoraria, advisory boards). L.A.V. reports grants from the Else-Kroener-Fresenius Stiftung (2015_A224) and speaker honoraria from Sanofi-Genzyme. S.B. receives grant support from the German Research Foundation (BR4917/3). J.M. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria, advisory boards). S.P. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria, advisory boards). R.W. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria, advisory boards, educational materials and review writing). W.J.J. received speaker honoraria from Alexion and Ablynx (advisory boards). W.M. received honoraria from Ablynx, Takeda, and Shire (speaker honoraria, advisory boards). M.T. reports research grants from the Sonnenfeld Stiftung, Charité3R, and Novartis and personal fees from Baxter, Cytosorbents, Novartis, and Takeda (speaker honoraria, advisory boards). M.R. reports personal fees from Alexion, Diamed, Roche, and Sanofi Genzyme (speaker honoraria, travel support). A. Gäckler reports personal fees from Alexion and Ablynx (speaker honoraria, advisory boards). F.B. received honoraria from Amgen, Sanofi, Akcea, Hexal, MSD, AstraZeneca, Alexion, and Astellas (speaker honoraria, advisory boards). U.S. reports study fees, travel support and consultancy fees from Alexion and study fees and consultancy fees Ablynx. M. Bommer has received honoraria from Ablynx, Alexion, Sanofi, and Amgen, as well as study fees from Ablynx and Amgen. C.v.A. reports personal fees from Sanofi Genzyme (advisory boards). The remaining authors declare no competing financial interests.
Figures

References
-
- Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129(21):2836-2846. - PubMed
-
- Peyvandi F, Scully M, Kremer Hovinga JA, et al. ; TITAN Investigators . Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511-522. - PubMed
-
- Scully M, Cataland SR, Peyvandi F, et al. ; HERCULES Investigators . Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335-346. - PubMed
-
- Bendapudi PK, Hurwitz S, Fry A, et al. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4(4):e157-e164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

