ADAMTS13 and VWF activities guide individualized caplacizumab treatment in patients with aTTP
- PMID: 32634237
- PMCID: PMC7362349
- DOI: 10.1182/bloodadvances.2020001987
ADAMTS13 and VWF activities guide individualized caplacizumab treatment in patients with aTTP
Abstract
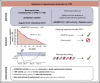
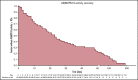
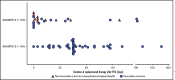
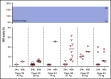
Introduction of the nanobody caplacizumab was shown to be effective in the treatment of acquired thrombotic thrombocytopenic purpura (aTTP) in the acute setting. The official recommendations include plasma exchange (PEX), immunosuppression, and the use of caplacizumab for a minimum of 30 days after stopping daily PEX. This study was a retrospective, observational analysis of the use of caplacizumab in 60 patients from 29 medical centers in Germany. Immunosuppressive treatment led to a rapid normalization of ADAMTS13 activities (calculated median, 21 days). In 35 of 60 patients, ADAMTS13 activities started to normalize before day 30 after PEX; in 11 of 60 patients, the treatment was extended beyond day 30; and in 5 patients, it was extended even beyond day 58 due to persistent autoimmune activity. In 34 of 60 instances, caplacizumab was stopped before day 30 with a favorable outcome whenever ADAMTS13 activities were >10%. In contrast, 11 of 34 patients with ADAMTS13 activities <10% at the time of stopping caplacizumab treatment developed a nonfavorable outcome (disease exacerbation or relapse). In some cases, prolongation of the treatment interval to every other day was feasible and resulted in a sustained reduction of von Willebrand factor activity. ADAMTS13 activity measurements are central for a rapid diagnosis in the acute setting but also to tailor disease management. An ADAMTS13 activity-guided approach seems safe for identifying the individual time point when to stop caplacizumab to prevent overtreatment and undertreatment; this approach will result in significant cost savings without jeopardizing the well-being of patients. In addition, von Willebrand factor activity may serve as a biomarker for drug monitoring.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.T.B. reports grants from the German Research Foundation (BR2955/8) during the conduct of the study; and personal fees from Alexion, Astellas, Bayer, Sanofi Genzyme, Pfizer, and Vifor (speaker honoraria and advisory boards). L.A.V. reports grants from the Else-Kroener-Fresenius Stiftung (2015_A224); and speaker honoraria from Sanofi Genzyme. S.B. receives grant support from the German Research Foundation (BR4917/3). J.M. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria and advisory boards). S.A.P. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria and advisory boards). R.W. received personal fees from Alexion, Sanofi Genzyme, and Ablynx (speaker honoraria, advisory boards, educational materials, and review writing). W.J.J. received speaker honoraria from Alexion and Ablynx (advisory boards). W.M. received honoraria from Ablynx, Takeda, and Shire (speaker honoraria and advisory boards). M.T. reports research grants from the Sonnenfeld Stiftung, Charité3R, and Novartis; and personal fees from Baxter, Cytosorbents, Novartis, and Takeda (speaker honoraria and advisory boards). M.R. reports personal fees from Alexion, Diamed, Roche, and Sanofi Genzyme (speaker honoraria and travel support). A. Gäckler reports personal fees from Alexion and Ablynx (speaker honoraria and advisory boards), F.B. received honoraria from Amgen, Sanofi, Akcea, Hexal, MSD, AstraZeneca, Alexion, and Astellas (speaker honoraria and advisory boards). U.S. reports study fees, travel support, and consultancy fees from Alexion; and study fees and consultancy fees Ablynx. M. Bommer received honoraria from Ablynx, Alexion, Amgen, and Sanofi; and study fees from Ablynx and Amgen. C.v.A. reports personal fees from Sanofi Genzyme (advisory boards). The remaining authors declare no competing financial interests.
Figures
Similar articles
-
A critical evaluation of caplacizumab for the treatment of acquired thrombotic thrombocytopenic purpura.Expert Rev Hematol. 2020 Nov;13(11):1153-1164. doi: 10.1080/17474086.2020.1819230. Epub 2020 Oct 12. Expert Rev Hematol. 2020. PMID: 32876503 Review.
-
Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura.J Thromb Haemost. 2017 Jul;15(7):1448-1452. doi: 10.1111/jth.13716. Epub 2017 Jun 5. J Thromb Haemost. 2017. PMID: 28445600 Clinical Trial.
-
Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura.Blood Adv. 2020 Jul 14;4(13):3085-3092. doi: 10.1182/bloodadvances.2020001973. Blood Adv. 2020. PMID: 32634236 Free PMC article.
-
Caplacizumab as an emerging treatment option for acquired thrombotic thrombocytopenic purpura.Drug Des Devel Ther. 2019 Apr 17;13:1251-1258. doi: 10.2147/DDDT.S134470. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31118566 Free PMC article. Review.
-
Delayed normalization of ADAMTS13 activity in acute thrombotic thrombocytopenic purpura in the caplacizumab era.Blood. 2023 May 4;141(18):2206-2213. doi: 10.1182/blood.2022018847. Blood. 2023. PMID: 36720103
Cited by
-
Immune thrombotic thrombocytopenic purpura: Personalized therapy using ADAMTS-13 activity and autoantibodies.Res Pract Thromb Haemost. 2021 Dec 9;5(8):e12606. doi: 10.1002/rth2.12606. eCollection 2021 Dec. Res Pract Thromb Haemost. 2021. PMID: 34938937 Free PMC article.
-
Concomitant presentation of thrombotic thrombocytopenic purpura, immune thrombocytopenia, and autoimmune hemolytic anemia in a patient with newly diagnosed systemic lupus erythematosus.Clin Nephrol Case Stud. 2023 Dec 12;11:147-153. doi: 10.5414/CNCS111193. eCollection 2023. Clin Nephrol Case Stud. 2023. PMID: 38170038 Free PMC article.
-
Evidence-Based Minireview: Should caplacizumab be used routinely in unselected patients with immune thrombotic thrombocytopenic purpura?Hematology Am Soc Hematol Educ Program. 2022 Dec 9;2022(1):491-494. doi: 10.1182/hematology.2022000412. Hematology Am Soc Hematol Educ Program. 2022. PMID: 36485149 Free PMC article. No abstract available.
-
TTP: From empiricism for an enigmatic disease to targeted molecular therapies.Br J Haematol. 2022 Apr;197(2):156-170. doi: 10.1111/bjh.18040. Epub 2022 Feb 10. Br J Haematol. 2022. PMID: 35146746 Free PMC article. Review.
-
Real-World Data Analysis of Patients Affected by Immune-Mediated Thrombotic Thrombocytopenic Purpura in Italy.J Clin Med. 2024 Feb 27;13(5):1342. doi: 10.3390/jcm13051342. J Clin Med. 2024. PMID: 38592185 Free PMC article.
References
-
- Mazepa MA, Masias C, Chaturvedi S. How targeted therapy disrupts the treatment paradigm for acquired TTP: the risks, benefits, and unknowns. Blood. 2019;134(5):415-420. - PubMed
-
- Peyvandi F, Scully M, Kremer Hovinga JA, et al. ; TITAN Investigators . Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511-522. - PubMed
-
- Scully M, Cataland SR, Peyvandi F, et al. ; HERCULES Investigators . Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335-346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

