A potentially effective treatment for COVID-19: A systematic review and meta-analysis of convalescent plasma therapy in treating severe infectious disease
- PMID: 32634589
- PMCID: PMC7334933
- DOI: 10.1016/j.ijid.2020.06.107
A potentially effective treatment for COVID-19: A systematic review and meta-analysis of convalescent plasma therapy in treating severe infectious disease
Abstract
Background: Convalescent plasma (CP) has been used successfully to treat many types of infectious disease, and has shown initial effects in the treatment of the emerging 2019 coronavirus disease (COVID-19). However, its curative effects and feasibility have yet to be confirmed by formal evaluation and well-designed clinical trials. To explore the effectiveness of treatment and predict the potential effects of CP with COVID-19, studies of different types of infectious disease treated with CP were included in this systematic review and meta-analysis.
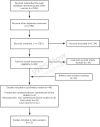
Methods: Related studies were obtained from databases and screened according to the inclusion criteria. The data quality was assessed, and the data were extracted and pooled for analysis.
Results: 40 studies on CP treatment for infectious diseases were included. Our study found that CP treatment could reduce the risk of mortality, with a low incidence of adverse events, promote the production of antibodies, lead to a decline in viral load, and shorten the disease course. A meta-analysis of 15 controlled studies showed that there was a significantly lower mortality rate in the group treated with CP (pooled OR=0.32; 95% CI=0.19-0.52; p<0.001, I2=54%) compared with the control groups. Studies were mostly of low or very low quality, with a moderate or high risk of bias. The sources of clinical and methodological heterogeneity were identified. The exclusion of heterogeneity indicated that the results were stable.
Conclusions: CP therapy has some curative effect and is well tolerated in treating infectious diseases. It is a potentially effective treatment for COVID-19.
Keywords: Convalescent plasma (CP); Coronavirus disease 2019 (COVID-19); Infectious disease; Meta-analysis.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors report no declarations of interest.
Figures


References
-
- Bang O. Convalescent serum in the treatment of influenza pneumonia. Nor Mag Laegevidenskaben. 1920;81:255–261.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

