A Review of Topical Phage Therapy for Chronically Infected Wounds and Preparations for a Randomized Adaptive Clinical Trial Evaluating Topical Phage Therapy in Chronically Infected Diabetic Foot Ulcers
- PMID: 32635429
- PMCID: PMC7400337
- DOI: 10.3390/antibiotics9070377
A Review of Topical Phage Therapy for Chronically Infected Wounds and Preparations for a Randomized Adaptive Clinical Trial Evaluating Topical Phage Therapy in Chronically Infected Diabetic Foot Ulcers
Abstract
The advent and increasing prevalence of antimicrobial resistance commensurate with the absence of novel antibiotics on the horizon raises the specter of untreatable infections. Phages have been safely administered to thousands of patients exhibiting signals of efficacy in many experiencing infections refractory to antecedent antibiotics. Topical phage therapy may represent a convenient and efficacious treatment modality for chronic refractory infected cutaneous wounds spanning all classifications including venous stasis, burn-mediated, and diabetic ulcers. We will initially provide results from a systematic literature review of topical phage therapy used clinically in refractorily infected chronic wounds. We will then segue into a synopsis of the preparations for a forthcoming phase II a randomized placebo-controlled clinical trial assessing the therapeutic efficacy exploiting adjunctive personalized phage administration, delivered topically, intravenously (IV) and via a combination of both modalities (IV + topical) in the treatment of infected diabetic foot ulcers (perhaps the canonical paradigm representing complicated recalcitrant infected cutaneous wounds).
Keywords: and antibiotics; bacteriophage; infected ulcers; infected wounds; phage library; phage-antibiotic synergy; phage-phage synergy; topical; topical therapeutics; ulcers; wounds.
Conflict of interest statement
The authors declare that they have no competing or conflict of interest.
Figures

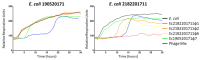

References
-
- World Health Organization . Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; Geneva, Switzerland: 2014. [(accessed on 1 May 2019)]. pp. 1–257. Available online: http://www.who.int.
-
- Davies E.V., James C.E., Williams D., O’Brien S., Fothergill J.L., Haldenby S., Paterson S., Winstanley C., Brockhurst M.A. Temperate phages both mediate and drive adaptive evolution in pathogen biofilms. Proc. Natl. Acad. Sci. USA. 2016;113:8266–8271. doi: 10.1073/pnas.1520056113. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

