Impact of Case Definitions on Efficacy Estimation in Clinical Trials-A Proof-of-Principle Based on Historical Examples
- PMID: 32635553
- PMCID: PMC7400704
- DOI: 10.3390/antibiotics9070379
Impact of Case Definitions on Efficacy Estimation in Clinical Trials-A Proof-of-Principle Based on Historical Examples
Abstract
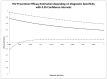
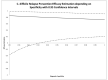
Efficacy estimations in clinical trials are based on case definitions. Commonly, they are a more or less complex set of conditions that have to be fulfilled in order to define a clinical case. In the simplest variant, such a case is identical with a single positive diagnostic test result. Frequently, however, case definitions are more complex. Further, their conditions often ignore the inherent logical structure of symptoms and disease: A symptom or a set of symptoms may be necessary but not sufficient for the unambiguous identification of a case. After describing the structure of case definitions and its impact on efficacy estimations, we exemplify this impact using data from two clinical trials dealing with the effectiveness of the vaginal application of tenofovir gel for the prevention of HIV infections and with the therapeutic effects of fecal transplantation on recurrent Clostridium difficile infections. We demonstrate that the diagnostic performance of case definitions affects efficacy estimations for interventions in clinical trials. The potential risk of bias and uncertainty is high, irrespective of the complexity of the case definition. Accordingly, case definitions in clinical trials should focus on specificity in order to avoid the risk of bias.
Keywords: case definition; clinical trial; efficacy estimation; specificity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Impact of diagnostic methods on efficacy estimation - a proof-of-principle based on historical examples.Trop Med Int Health. 2020 Mar;25(3):357-363. doi: 10.1111/tmi.13351. Epub 2019 Dec 9. Trop Med Int Health. 2020. PMID: 31758838
-
Diagnostic accuracy of three clinical case definitions for advanced HIV disease.AIDS. 1992 Mar;6(3):295-9. doi: 10.1097/00002030-199203000-00006. AIDS. 1992. PMID: 1348946
-
Comparison of alternative biochemical failure definitions based on clinical outcome in 4839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995.Int J Radiat Oncol Biol Phys. 2003 Nov 15;57(4):929-43. doi: 10.1016/s0360-3016(03)00631-x. Int J Radiat Oncol Biol Phys. 2003. PMID: 14575823
-
[Fecal microbiota transplantation in recurrent Clostridium difficile infections. Framework and pharmaceutical preparation aspects].Ann Pharm Fr. 2015 Sep;73(5):323-31. doi: 10.1016/j.pharma.2015.02.004. Epub 2015 Mar 29. Ann Pharm Fr. 2015. PMID: 25825054 Review. French.
-
Fecal Microbiota Transplantation in Recurrent Clostridium Difficile Infection: Is it Superior to Other Conventional Methods?Cureus. 2020 Aug 11;12(8):e9653. doi: 10.7759/cureus.9653. Cureus. 2020. PMID: 32923252 Free PMC article. Review.
Cited by
-
Antimicrobial Resistance Patterns in Clostridioides difficile Strains Isolated from Neonates in Germany.Antibiotics (Basel). 2020 Aug 4;9(8):481. doi: 10.3390/antibiotics9080481. Antibiotics (Basel). 2020. PMID: 32759868 Free PMC article.
-
Optimization of Case Definitions for Sensitivity as a Preventive Strategy-A Modelling Exemplified with Rapid Diagnostic Test-Based Prevention of Sexual HIV Transmission.Diagnostics (Basel). 2021 Nov 10;11(11):2079. doi: 10.3390/diagnostics11112079. Diagnostics (Basel). 2021. PMID: 34829425 Free PMC article.
References
-
- Abdool Karim Q., Abdool Karim S.S., Frohlich J.A., Grobler A.C., Baxter C., Mansoor L.E., Kharsany A.B., Sibeko S., Mlisana K.P., Omar Z., et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

