Population pharmacokinetics of vedolizumab in Asian and non-Asian patients with ulcerative colitis and Crohn's disease
- PMID: 32635680
- PMCID: PMC7873400
- DOI: 10.5217/ir.2019.09167
Population pharmacokinetics of vedolizumab in Asian and non-Asian patients with ulcerative colitis and Crohn's disease
Abstract
Background/aims: Vedolizumab is indicated for moderately-to-severely active ulcerative colitis (UC) and Crohn's disease (CD). Because multiple factors may result in different pharmacokinetics and clinical efficacies, understanding determinants of vedolizumab clearance may enhance dose and treatment strategies. The aim was to characterize vedolizumab pharmacokinetics in Asian and non-Asian UC and CD patients.
Methods: Population pharmacokinetic analysis for repeated measures, using data from 5 studies, was conducted using nonlinear mixed-effects modeling. A Bayesian estimation approach in NONMEM 7.3 was utilized to leverage the predominantly sparse data available for this analysis with results from a prior population pharmacokinetic analysis of vedolizumab.
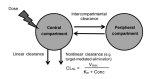
Results: Vedolizumab pharmacokinetics were described by a 2-compartment model with parallel linear and nonlinear elimination. Using reference covariate values, linear elimination half life of vedolizumab was 24.7 days for anti-vedolizumab antibody (AVA)-negative patients and 18.1 days for AVA-positive patients; linear clearance (CLL) was 0.165 L/day for AVA-negative patients and 0.246 L/day for AVA-positive patients; central (Vc) and peripheral compartment volumes of distribution were 3.16 L and 1.84 L, respectively. Interindividual variabilities (percent coefficient of variation) were 30.8% for CLL and 19% for Vc; interoccasion variability on CLL was 20.3%; residual variance was 17.8%. For albumin, body weight and AVA, only extreme values were identified as potentially clinically important predictors of CLL. The effect of race (Asian/non-Asian) and diagnosis (UC/CD) on CLL was negligible and likely not of clinical importance.
Conclusions: Pharmacokinetic parameters were similar in Asian and non-Asian patients with moderately-to-severely active UC and CD. This analysis supports use of vedolizumab flat-fixed dosing in these patients. (Clinicaltrials.gov Identifiers: NCT00783718 (GEMINI 1); NCT00783692 (GEMINI 2). CCT 101; NCT02039505 and CCT-001; NCT02038920).
Keywords: Asian; Colitis, ulcerative; Crohn disease; Population pharmacokinetics; Vedolizumab.
Conflict of interest statement
Okamoto H is an employee of Takeda PRA Development Center, KK, Osaka, Japan. Rosario M is an employee of Takeda Development Center Americas Inc, Cambridge, MA, USA and holds equity stake in Takeda. Hori T is an employee of Takeda Pharmaceutical Company Limited, Osaka, Japan and holds equity stake in Takeda. Dirks NL is an employee of Metrum Research Group, Tariffville, CT, USA and was a paid consultant to Takeda for this work. Hibi T is an employee of Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Japan, and has received honoraria from Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, AbbVie GK, Zeria Pharmaceutical, JIMRO, Aspen Japan K.K, Ferring, Gilead Sciences, Kissei Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Janssen Pharmaceutical and Pfizer Japan; has received scholarship grants from EA Pharma, Abbvie, JIMRO, Zeria Pharmaceutical and Otsuka Holdings. No other potential conflict of interest relevant to this article was reported.
Hibi T is an editorial board member of the journal but did not involve in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Figures

References
-
- Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–875. - PubMed
-
- U.S. Food and Drug Administration Takeda Pharmaceuticals America Inc. Entyvio (vedolizumab) prescribing information [Internet] c2014 [cited 2019 May 10]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125476s000lbl.pdf.
-
- European Medicine Agency Takeda Pharma A/S. Entyvio (vedolizumab) summary of product characteristics [Internet] 2019 c2018 [cited 2019 May 10]. https://www.ema.europa.eu/en/documents/product-information/entyvio-epar-....
-
- Zhu Y, Hu C, Lu M, et al. Population pharmacokinetic modeling of ustekinumab, a human monoclonal antibody targeting IL-12/23p40, in patients with moderate to severe plaque psoriasis. J Clin Pharmacol. 2009;49:162–175. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

