miR-96-5p regulated TGF-β/SMAD signaling pathway and suppressed endometrial cell viability and migration via targeting TGFBR1
- PMID: 32635855
- PMCID: PMC7469441
- DOI: 10.1080/15384101.2020.1777804
miR-96-5p regulated TGF-β/SMAD signaling pathway and suppressed endometrial cell viability and migration via targeting TGFBR1
Retraction in
-
Statement of Retraction: miR-96-5p regulated TGF-β/SMAD signaling pathway and suppressed endometrial cell viability and migration via targeting TGFBR1.Cell Cycle. 2024 Jul-Aug;23(13-16):i. doi: 10.1080/15384101.2024.2370717. Epub 2024 Jul 4. Cell Cycle. 2024. PMID: 38963704 Free PMC article. No abstract available.
Abstract
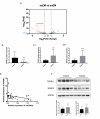
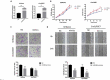
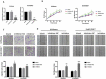
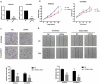
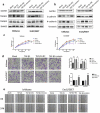
We previously performed high throughput RNA-seq in paired eutopic and ectopic endometrial specimen of endometriosis patients, and validated the results by qRT-PCR in endometriosis endometrial tissues. MiR-96-5p was significantly downregulated in ectopic endometrial tissues compared to eutopic tissues. In order to identify the role of miR-96-5p in endometriosis and endometrial cells, and investigate the underlying mechanisms, the Ishikawa and End1/E6E7 cell lines were transfected with miR-96-5p mimics, miR-96-5p inhibitors or TGFBR1 siRNA. The expression of TGF-β/SMAD signaling pathway components and epithelial-mesenchymal transition (EMT) markers were examined by qRT-PCR and western blot, and cell viability and migration were determined by CCK-8, transwell and wound healing assays, respectively. We discovered miR-96-5p to be significantly downregulated while TGFBR1 was distinctly up-regulated in endometriosis. Overexpression of miR-96-5p inhibited endometrial cells viability and migration, while inhibition of miR-96-5p had opposite effect. Furthermore, we confirmed TGFBR1 was a direct target of miR-96-5p. Overexpression of miR-96-5p could block the TGF-β/SMAD signaling pathway via targeting TGFBR1 and reverse the TGF-β1 induced EMT in endometrial cell lines. In conclusion, we demonstrated that miR-96-5p interacted with TGF-β/SMAD signaling pathway and blocked the TGF-β1 induced EMT in endometrial cells via directly targeting TGFBR1.
Keywords: EMT; TGF-β/SMAD signaling pathway; TGFBR1; endometriosis; miR-96-5p.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures


References
-
- Giudice LC, Kao LC.. Endometriosis [J]. Lancet. 2004;364(9447):1789–1799. - PubMed
-
- Shafrir AL, Farland LV, Shah DK, et al. Risk for and consequences of endometriosis: A critical epidemiologic review [J]. Best Pract Res Clin Obstetrics Gynaecol. 2018. August;51(51):1–15. - PubMed
-
- Tomassetti C, D’Hooghe T. Endometriosis and infertility: insights into the causal link and management strategies [J]. Best Pract Res Clin Obstetrics Gynaecol. 2018. August;51(51):25–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
