Clinical Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-qPCR), Direct RT-qPCR, Reverse Transcription-Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test To Diagnose COVID-19
- PMID: 32636214
- PMCID: PMC7448663
- DOI: 10.1128/JCM.01438-20
Clinical Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-qPCR), Direct RT-qPCR, Reverse Transcription-Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test To Diagnose COVID-19
Abstract
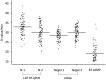
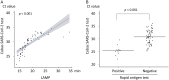
The clinical performances of six molecular diagnostic tests and a rapid antigen test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were clinically evaluated for the diagnosis of coronavirus disease 2019 (COVID-19) in self-collected saliva. Saliva samples from 103 patients with laboratory-confirmed COVID-19 (15 asymptomatic and 88 symptomatic) were collected on the day of hospital admission. SARS-CoV-2 RNA in saliva was detected using a quantitative reverse transcription-PCR (RT-qPCR) laboratory-developed test (LDT), a cobas SARS-CoV-2 high-throughput system, three direct RT-qPCR kits, and reverse transcription-loop-mediated isothermal amplification (RT-LAMP). The viral antigen was detected by a rapid antigen immunochromatographic assay. Of the 103 samples, viral RNA was detected in 50.5 to 81.6% of the specimens by molecular diagnostic tests, and an antigen was detected in 11.7% of the specimens by the rapid antigen test. Viral RNA was detected at significantly higher percentages (65.6 to 93.4%) in specimens collected within 9 days of symptom onset than in specimens collected after at least 10 days of symptoms (22.2 to 66.7%) and in specimens collected from asymptomatic patients (40.0 to 66.7%). Self-collected saliva is an alternative specimen option for diagnosing COVID-19. The RT-qPCR LDT, a cobas SARS-CoV-2 high-throughput system, direct RT-qPCR kits (except for one commercial kit), and RT-LAMP showed sufficient sensitivities in clinical use to be selectively used in clinical settings and facilities. The rapid antigen test alone is not recommended for an initial COVID-19 diagnosis because of its low sensitivity.
Keywords: RT-LAMP; RT-qPCR; SARS-CoV-2; antigen test; saliva.
Copyright © 2020 Nagura-Ikeda et al.
Figures
References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. doi:10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- Kitagawa Y, Orihara Y, Kawamura R, Imai K, Sakai J, Tarumoto N, Matsuoka M, Takeuchi S, Maesaki S, Maeda T. 2020. Evaluation of rapid diagnosis of novel coronavirus disease (COVID-19) using loop-mediated isothermal amplification. J Clin Virol 129:104446. doi:10.1016/j.jcv.2020.104446. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

