LACTB Regulates PIK3R3 to Promote Autophagy and Inhibit EMT and Proliferation Through the PI3K/AKT/mTOR Signaling Pathway in Colorectal Cancer
- PMID: 32636680
- PMCID: PMC7335311
- DOI: 10.2147/CMAR.S250661
LACTB Regulates PIK3R3 to Promote Autophagy and Inhibit EMT and Proliferation Through the PI3K/AKT/mTOR Signaling Pathway in Colorectal Cancer
Abstract
Background: Colorectal cancer (CRC) is one of the most common aggressive malignancies. LACTB functions as a tumor suppressor, and previous findings have demonstrated that LACTB can inhibit epithelial-to-mesenchymal transition (EMT) and proliferation of breast cancer and CRC cells. However, few studies have investigated the roles of LACTB in autophagy and proliferation in CRC. The current study aimed to identify the roles of LACTB in EMT and proliferation associated with autophagy in CRC and to elucidate the probable molecular mechanisms through which LACTB are involved in these processes.
Materials and methods: Transwell invasion, MTT, transmission electron microscopy, RNA-seq, immunoprecipitation, immunohistochemistry and Western blotting assays were performed to evaluate the migratory, invasive, proliferative and autophagic abilities of CRC cells, and the levels of active molecules involved in PI3K/AKT signaling were examined through Western blotting analysis. In addition, the in vivo function of LACTB was assessed using a tumor xenograft model.
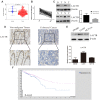
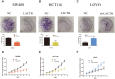
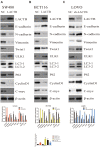
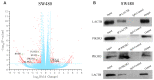
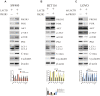
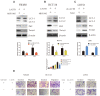
Results: Weaker LACTB expression was found in CRC tissue samples than in nonmalignant tissue samples, and LACTB inhibited cell invasion, migration, and proliferation by promoting autophagy in vitro. Furthermore, the regulatory effects of LACTB on autophagy and EMT were partially attributed to the PI3K/AKT signaling pathway. The in vivo results also showed that LACTB modulated CRC tumorigenesis.
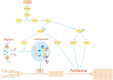
Conclusion: LACTB can regulate the activity of PIK3R3 to influence the level of PI3K, and it also promotes autophagy and inhibits EMT and proliferation in part through the PI3K/AKT/mTOR signaling pathway.
Keywords: EMT; LACTB; PIK3R3; autophagy; colorectal cancer; proliferation.
© 2020 Xu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Kahi CJ, Boland CR, Dominitz JA, et al. United states multi-society task force on colorectal, colonoscopy surveillance after colorectal cancer resection: recommendations of the us multi-society task force on colorectal cancer. Gastroenterology. 2016;150(3):758–768.e711. doi: 10.1053/j.gastro.2016.01.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

