A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic
- PMID: 32636754
- PMCID: PMC7317023
- DOI: 10.3389/fphar.2020.00937
A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic
Abstract
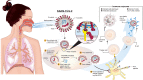
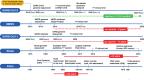
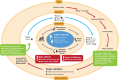
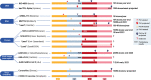
A novel coronavirus SARS-CoV-2 causing Coronavirus disease 2019 (COVID-19) has entered the human population and has spread rapidly around the world in the first half of 2020 causing a global pandemic. The virus uses its spike glycoprotein receptor-binding domain to interact with host cell angiotensin-converting enzyme 2 (ACE2) sites to initiate a cascade of events that culminate in severe acute respiratory syndrome in some individuals. In efforts to curtail viral spread, authorities initiated far-reaching lockdowns that have disrupted global economies. The scientific and medical communities are mounting serious efforts to limit this pandemic and subsequent waves of viral spread by developing preventative vaccines and repurposing existing drugs as potential therapies. In this review, we focus on the latest developments in COVID-19 vaccine development, including results of the first Phase I clinical trials and describe a number of the early candidates that are emerging in the field. We seek to provide a balanced coverage of the seven main platforms used in vaccine development that will lead to a desired target product profile for the "ideal" vaccine. Using tales of past vaccine discovery efforts that have taken many years or that have failed, we temper over exuberant enthusiasm with cautious optimism that the global medical community will reach the elusive target to treat COVID-19 and end the pandemic.
Keywords: COVID-19; SARS-CoV-2; clinical trial; coronavirus; immune response; public health; vaccine.
Copyright © 2020 Funk, Laferrière and Ardakani.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

