Pathophysiology of paraneoplastic and autoimmune encephalitis: genes, infections, and checkpoint inhibitors
- PMID: 32636932
- PMCID: PMC7318829
- DOI: 10.1177/1756286420932797
Pathophysiology of paraneoplastic and autoimmune encephalitis: genes, infections, and checkpoint inhibitors
Abstract
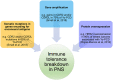
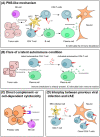
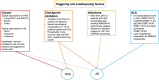
Paraneoplastic neurological syndromes (PNSs) are rare complications of systemic cancers that can affect all parts of the central and/or peripheral nervous system. A body of experimental and clinical data has demonstrated that the pathogenesis of PNSs is immune-mediated. Nevertheless, the mechanisms leading to immune tolerance breakdown in these conditions remain to be elucidated. Despite their rarity, PNSs offer a unique perspective to understand the complex interplay between cancer immunity, effect of immune checkpoint inhibitors (ICIs), and mechanisms underlying the attack of neurons in antibody-mediated neurological disorders, with potentially relevant therapeutic implications. In particular, it is reported that ICI treatment can unleash PNSs and that the immunopathological features of PNS-related tumors are distinctive, showing prominent tumor-infiltrating lymphocytes and germinal center reactions. Intriguingly, similar pathological substrates have gained further attention as potential biomarkers of ICI-sensitivity and oncological prognosis. Moreover, the genetic analysis of PNS-associated tumors has revealed specific molecular signatures and mutations in genes encoding onconeural proteins, leading to the production of highly immunogenic neoantigens. Other than PNSs, autoimmune encephalitides (AEs) comprise a recently described group of disorders characterized by prominent neuropsychiatric symptoms, diverse antibody spectrum, and less tight association with cancer. Other triggering factors seem to be involved in AEs. Recent data have shed light on the importance of preceding infections (in particular, herpes simplex virus encephalitis) in inducing neurological autoimmune disorders in susceptible individuals (those with a selective deficiency in the innate immune system). In addition, in some AEs (e.g. LGI1-antibody encephalitis) an association with specific host-related factors [e.g., human leukocyte antigen (HLA)] was clearly demonstrated. We provide herein a comprehensive review of the most recent findings in the field of PNSs and AEs, with particular focus on their triggering factors and immunopathogenesis.
Keywords: CTLA-4; HLA; NMDAR; PD-1; autoimmune encephalitis; immune checkpoint inhibitors; neurological adverse events; paraneoplastic neurological syndromes.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: AV reported receiving a fellowship grant from the European Academy of Neurology (EAN). No other disclosures were reported.
Figures
Similar articles
-
Paraneoplastic Autoimmune Neurological Syndromes and the Role of Immune Checkpoint Inhibitors.Neurotherapeutics. 2022 Apr;19(3):848-863. doi: 10.1007/s13311-022-01184-0. Epub 2022 Jan 18. Neurotherapeutics. 2022. PMID: 35043373 Free PMC article. Review.
-
Paraneoplastic neurological syndromes in the era of immune-checkpoint inhibitors.Nat Rev Clin Oncol. 2019 Sep;16(9):535-548. doi: 10.1038/s41571-019-0194-4. Nat Rev Clin Oncol. 2019. PMID: 30867573 Review.
-
Ovarian Teratoma-Related Paraneoplastic Neurological Syndromes.Front Oncol. 2022 May 16;12:892539. doi: 10.3389/fonc.2022.892539. eCollection 2022. Front Oncol. 2022. PMID: 35651803 Free PMC article. Review.
-
Paraneoplastic neurological syndrome: growing spectrum and relevance.Neurol Sci. 2022 Jun;43(6):3583-3594. doi: 10.1007/s10072-022-06083-y. Epub 2022 Apr 23. Neurol Sci. 2022. PMID: 35460452 Review.
-
Pathogenesis, diagnosis and treatment of paraneoplastic neurologic syndromes.Expert Rev Neurother. 2021 Jun;21(6):675-686. doi: 10.1080/14737175.2021.1927713. Epub 2021 May 27. Expert Rev Neurother. 2021. PMID: 33960258 Review.
Cited by
-
Evaluation and management of acute high-grade immunotherapy-related neurotoxicity.Heliyon. 2023 Feb 14;9(3):e13725. doi: 10.1016/j.heliyon.2023.e13725. eCollection 2023 Mar. Heliyon. 2023. PMID: 36851967 Free PMC article. Review.
-
How to diagnose and manage neurological toxicities of immune checkpoint inhibitors: an update.J Neurol. 2022 Mar;269(3):1701-1714. doi: 10.1007/s00415-021-10870-6. Epub 2021 Oct 27. J Neurol. 2022. PMID: 34708250
-
[(Auto)immunity in focal epilepsy: mechanisms of (auto‑)immune-inflammatory epileptogenic neurodegeneration].Nervenarzt. 2024 Oct;95(10):932-937. doi: 10.1007/s00115-024-01695-5. Epub 2024 Jul 2. Nervenarzt. 2024. PMID: 38953922 Free PMC article. Review. German.
-
Back to the future: encephalitis lethargica as an autoimmune disorder?Neurol Sci. 2024 Jan;45(1):93-99. doi: 10.1007/s10072-023-07053-8. Epub 2023 Sep 9. Neurol Sci. 2024. PMID: 37688743 Review.
-
Case Report: Autoimmune Psychosis in Chromosome 22q11.2 Deletion Syndrome.Front Immunol. 2021 Oct 14;12:708625. doi: 10.3389/fimmu.2021.708625. eCollection 2021. Front Immunol. 2021. PMID: 34721378 Free PMC article.
References
-
- Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med 2018; 378: 840–851. - PubMed
-
- Alexopoulos H, Dalakas MC. The immunobiology of autoimmune encephalitides. J Autoimmun 2019; 104: 102339. - PubMed
-
- Joubert B, Dalmau J. The role of infections in autoimmune encephalitides. Rev Neurol (Paris) 2019; 175: 420–426. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

