CagA orchestrates eEF1A1 and PKCδ to induce interleukin-6 expression in Helicobacter pylori-infected gastric epithelial cells
- PMID: 32636937
- PMCID: PMC7333391
- DOI: 10.1186/s13099-020-00368-3
CagA orchestrates eEF1A1 and PKCδ to induce interleukin-6 expression in Helicobacter pylori-infected gastric epithelial cells
Abstract
Background: Helicobacter pylori colonises the stomach of approximately 50% of the global population. Cytotoxin-associated gene A protein (CagA) is one of the important virulent factors responsible for the increased inflammation and increases the risk of developing peptic ulcers and gastric carcinoma. The cytokine interleukin-6 (IL-6) has particularly important roles in the malignant transformation of gastric and intestinal epithelial cells as it is upregulated in H. pylori-infected gastric mucosa. In this study, we investigated the underlying mechanisms of CagA-induced IL-6 up-regulation during H. pylori infection. AGS cells, a human gastric adenocarcinoma cell line, lacking eEF1A1 were infected with CagA+ H. pylori (NCTC11637), CagA- H. pylori (NCTC11637ΔcagA), or transduced by Ad-cagA/Ad-GFP. The expression and production of IL-6 were measured by quantitative real-time reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay, respectively. The interactions among CagA, eukaryotic translation elongation factor 1-alpha 1 (eEF1A1), protein kinase Cδ (PKCδ), and signal transducer and activator of transcription 3 (STAT3) were determined by western blot or co-immunoprecipitation.
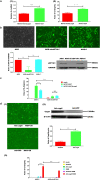
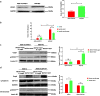
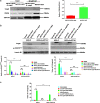
Results: During H. pylori infection, CagA-M (residues 256‒871aa) was found to interact with eEF1A1-I (residues 1‒240aa). NCTC11637 increased the expression of IL-6 in AGS cells compared with NCTC11637ΔcagA whereas knockdown of eEF1A1 in AGS cells completely abrogated these effects. Moreover, the CagA-eEF1A1 complex promoted the expression of IL-6 in AGS cells. CagA and eEF1A1 cooperated to mediate the expression of IL-6 by affecting the activity of p-STATS727 in the nucleus. Further, CagA-eEF1A1 affected the activity of STAT3 by recruiting PKCδ. However, blocking PKCδ inhibited the phosphorylation of STAT3S727 and induction of IL-6 by CagA.
Conclusions: CagA promotes the expression of IL-6 in AGS cells by recruiting PKCδ through eEF1A1 in the cytoplasm to increase the phosphorylation of STAT3S727 in the nucleus. These findings provide new insights into the function of CagA-eEF1A1 interaction in gastric adenocarcinoma.
Keywords: CagA; Gastric adenocarcinoma; Interleukin-6; PKCδ; eEF1A1; p-STAT3S727.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures


Similar articles
-
Helicobacter pylori CagA triggers expression of the bactericidal lectin REG3γ via gastric STAT3 activation.PLoS One. 2012;7(2):e30786. doi: 10.1371/journal.pone.0030786. Epub 2012 Feb 1. PLoS One. 2012. PMID: 22312430 Free PMC article.
-
CagA/cytotoxic strains of Helicobacter pylori and interleukin-8 in gastric epithelial cell lines.J Clin Pathol. 1994 Oct;47(10):945-50. doi: 10.1136/jcp.47.10.945. J Clin Pathol. 1994. PMID: 7962609 Free PMC article.
-
A novel role for Helicobacter pylori cytotoxin-associated gene A in negative regulation of autophagy in human gastric cells.BMC Gastroenterol. 2023 Sep 22;23(1):326. doi: 10.1186/s12876-023-02944-8. BMC Gastroenterol. 2023. PMID: 37740192 Free PMC article.
-
N-terminal region of Helicobacter pylori CagA induces IL-8 production in gastric epithelial cells via the β1 integrin receptor.J Med Microbiol. 2020 Mar;69(3):457-464. doi: 10.1099/jmm.0.001088. J Med Microbiol. 2020. PMID: 32100714
-
Jak1/Stat3 is an upstream signaling of NF-κB activation in Helicobacter pylori-induced IL-8 production in gastric epithelial AGS cells.Yonsei Med J. 2015 May;56(3):862-6. doi: 10.3349/ymj.2015.56.3.862. Yonsei Med J. 2015. PMID: 25837197 Free PMC article.
Cited by
-
The effect of the intratumoral microbiome on tumor occurrence, progression, prognosis and treatment.Front Immunol. 2022 Nov 18;13:1051987. doi: 10.3389/fimmu.2022.1051987. eCollection 2022. Front Immunol. 2022. PMID: 36466871 Free PMC article. Review.
-
Localization and Functional Roles of Components of the Translation Apparatus in the Eukaryotic Cell Nucleus.Cells. 2021 Nov 19;10(11):3239. doi: 10.3390/cells10113239. Cells. 2021. PMID: 34831461 Free PMC article. Review.
-
Association between IL-6 and prognosis of gastric cancer: a retrospective study.Therap Adv Gastroenterol. 2023 Nov 17;16:17562848231211543. doi: 10.1177/17562848231211543. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 38026103 Free PMC article.
-
Long-Term Culturing of FreeStyle 293-F Cells Affects Immunoglobulin G Glycome Composition.Biomolecules. 2023 Aug 14;13(8):1245. doi: 10.3390/biom13081245. Biomolecules. 2023. PMID: 37627310 Free PMC article.
-
A novel 3'tRNA-derived fragment tRF-Val promotes proliferation and inhibits apoptosis by targeting EEF1A1 in gastric cancer.Cell Death Dis. 2022 May 18;13(5):471. doi: 10.1038/s41419-022-04930-6. Cell Death Dis. 2022. PMID: 35585048 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

