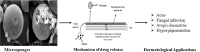
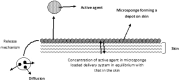
Microsponges for dermatological applications: Perspectives and challenges
- PMID: 32636947
- PMCID: PMC7327759
- DOI: 10.1016/j.ajps.2019.05.004
Microsponges for dermatological applications: Perspectives and challenges
Abstract
Dermatological disorders have a huge psychosocial impact, causing significant impairment of patient's life. Topical therapy plays a pivotal role in management of such disorders. Conventional topical delivery systems result in overmedication/undermedication, leading to adverse effects and reduction in therapeutic efficacy. Consequently, researchers have been striving towards the development of alternative delivery systems for dermatological applications. In the last decade, microsponges emerged as an attractive option for topical delivery. Their characteristic particle size offers enhanced benefits, making them superior to the contemporary microcarriers. The present review furnishes a comprehensive account of state of the art, important factors affecting the performance and mechanism of drug release from topically applied microsponges, along with characterization techniques. Further, a list of marketed products and their applications for common dermatological disorders has been presented. All in all, this paper is an attempt to lay a bibliographic foundation for researchers working in this field and foster further investigations in this arena.
Keywords: Dermatology; Drug release; Polymers; Safety aspects; Skin; Stability.
© 2019 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Figures
Similar articles
-
Therapeutic Application of Microsponges-based Drug Delivery Systems.Curr Pharm Des. 2022;28(8):595-608. doi: 10.2174/1381612828666220118121536. Curr Pharm Des. 2022. PMID: 35040411 Review.
-
Encapsulation of babchi essential oil into microsponges: Physicochemical properties, cytotoxic evaluation and anti-microbial activity.J Food Drug Anal. 2019 Jan;27(1):60-70. doi: 10.1016/j.jfda.2018.07.006. Epub 2018 Aug 16. J Food Drug Anal. 2019. PMID: 30648595 Free PMC article.
-
Microsponges based novel drug delivery system for augmented arthritis therapy.Saudi Pharm J. 2015 Oct;23(5):562-72. doi: 10.1016/j.jsps.2015.02.020. Epub 2015 Mar 7. Saudi Pharm J. 2015. PMID: 26594124 Free PMC article.
-
Development and Characterization of Polymeric Microsponge as a New Vehicle to Deliver Urea Topically.Recent Pat Nanotechnol. 2023;17(2):131-143. doi: 10.2174/1872210516666220422134046. Recent Pat Nanotechnol. 2023. PMID: 35466888
-
Microsponges: a futuristic approach for oral drug delivery.Expert Opin Drug Deliv. 2012 Jul;9(7):863-78. doi: 10.1517/17425247.2012.693072. Epub 2012 Jun 5. Expert Opin Drug Deliv. 2012. PMID: 22663167 Review.
Cited by
-
Cutaneous Pharmacokinetics of Topically Applied Novel Dermatological Formulations.AAPS PharmSciTech. 2024 Feb 27;25(3):46. doi: 10.1208/s12249-024-02763-4. AAPS PharmSciTech. 2024. PMID: 38413430 Review.
-
Preparation and Evaluation of a Microsponge Dermal Stratum Corneum Retention Drug Delivery System for Griseofulvin.AAPS PharmSciTech. 2022 Jul 19;23(6):199. doi: 10.1208/s12249-022-02362-1. AAPS PharmSciTech. 2022. PMID: 35854184
-
Resolving acne with optimized adapalene microspongeal gel, in vivo and clinical evaluations.Sci Rep. 2024 Jan 16;14(1):1359. doi: 10.1038/s41598-024-51392-1. Sci Rep. 2024. PMID: 38228631 Free PMC article.
-
Acne Vulgaris-Novel Treatment Options and Factors Affecting Therapy Adherence: A Narrative Review.J Clin Med. 2022 Dec 19;11(24):7535. doi: 10.3390/jcm11247535. J Clin Med. 2022. PMID: 36556150 Free PMC article. Review.
-
Pathology and Treatment of Psoriasis Using Nanoformulations.Biomedicines. 2023 May 30;11(6):1589. doi: 10.3390/biomedicines11061589. Biomedicines. 2023. PMID: 37371684 Free PMC article. Review.
References
-
- Carbone C., Leonardi A., Cupri S., Puglisi G., Pignatello R. Pharmaceutical and biomedical applications of lipid-based nanocarriers. Pharm Pat Anal. 2014;3(2):199–215. - PubMed
-
- Bagwe R.P., Kanicky J.R., Palla B.J., Patanjali P.K., Shah D.O. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit Rev Ther Drug Carrier Syst. 2001;18(1):77–140. - PubMed
-
- Bothorel P. Les microemulsions. L'Act Chim. 1985;(5):23–27.
-
- Wester R.C., Patel R., Nacht S., Leyden J., Melendres J., Maibach H. Controlled release of benzoyl peroxide from a porous microsphere polymeric system can reduce topical irritancy. J Am Acad Dermatol. 1991;24(5):720–726. - PubMed
Publication types
LinkOut - more resources
Full Text Sources