Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects
- PMID: 32636949
- PMCID: PMC7327776
- DOI: 10.1016/j.ajps.2019.06.003
Redox dual-stimuli responsive drug delivery systems for improving tumor-targeting ability and reducing adverse side effects
Abstract
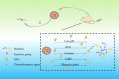
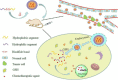
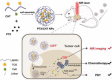
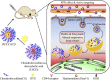
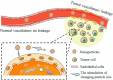
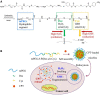
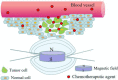
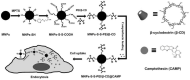
Cancer is a big challenge that has plagued the human beings for ages and one of the most effective treatments is chemotherapy. However, the low tumor-targeting ability limits the wide clinical application of chemotherapy. The microenvironment plays a critical role in many aspects of tumor genesis. It generates the tumor vasculature and it is highly implicated in the progression to metastasis. To maintain a suitable environment for tumor progression, there are special microenvironment in tumor cell, such as low pH, high level of glutathione (GSH) and reactive oxygen species (ROS), and more special enzymes, which is different to normal cell. Microenvironment-targeted therapy strategy could create new opportunities for therapeutic targeting. Compared to other targeting strategies, microenvironment-targeted therapy strategy will control the drug release into tumor cells more accurately. Redox responsive drug delivery systems (DDSs) are developed based on the high level of GSH in tumor cells. However, there are also GSH in normal cell though its level is lower. In order to control the release of drugs more accurately and reduce side effects, other drug release stimuli have been introduced to redox responsive DDSs. Under the synergistic reaction of two stimuli, redox dual-stimuli responsive DDSs will control the release of drugs more accurately and quickly and even increase the accumulation. This review summarizes strategies of redox dual-stimuli responsive DDSs such as pH, light, enzyme, ROS, and magnetic guide to delivery chemotherapeutic agents more accurately, aiming at providing new ideas for further promoting the drug release, enhancing tumor-targeting and improving anticancer effects. To better illustrate the redox dual-stimuli responsive DDS, preparations of carriers are also briefly described in the review.
Keywords: Chemotherapy; Drug delivery system; Dual-stimuli responsive; Redox responsive; Tumor-targeting.
© 2019 Published by Elsevier B.V. on behalf of Shenyang Pharmaceutical University.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Figures
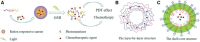
Similar articles
-
Stimuli-responsive drug delivery systems for head and neck cancer therapy.Drug Deliv. 2021 Dec;28(1):272-284. doi: 10.1080/10717544.2021.1876182. Drug Deliv. 2021. PMID: 33501883 Free PMC article. Review.
-
The Smart Dual-Stimuli Responsive Nanoparticles for Controlled Anti-Tumor Drug Release and Cancer Therapy.Anticancer Agents Med Chem. 2021;21(10):1202-1215. doi: 10.2174/1871520620666200924110418. Anticancer Agents Med Chem. 2021. PMID: 32972353 Review.
-
Smart Unimolecular Micelle-Based Polyprodrug with Dual-Redox Stimuli Response for Tumor Microenvironment: Enhanced in Vivo Delivery Efficiency and Tumor Penetration.ACS Appl Mater Interfaces. 2019 Oct 2;11(39):36130-36140. doi: 10.1021/acsami.9b13214. Epub 2019 Sep 18. ACS Appl Mater Interfaces. 2019. PMID: 31490659
-
Stimuli-responsive nanoscale drug delivery systems for cancer therapy.J Drug Target. 2019 Apr;27(4):423-433. doi: 10.1080/1061186X.2018.1519029. Epub 2019 Jan 24. J Drug Target. 2019. PMID: 30173577 Review.
-
Recent Advances in Metal-Organic Frameworks as Anticancer Drug Delivery Systems: A Review.Anticancer Agents Med Chem. 2021;21(18):2487-2504. doi: 10.2174/1871520621666210119093844. Anticancer Agents Med Chem. 2021. PMID: 33463479 Review.
Cited by
-
Advances in the stimuli-responsive mesoporous silica nanoparticles as drug delivery system nanotechnology for controlled release and cancer therapy.3 Biotech. 2023 Aug;13(8):274. doi: 10.1007/s13205-023-03651-7. Epub 2023 Jul 12. 3 Biotech. 2023. PMID: 37457870 Free PMC article. Review.
-
Aptamer-Functionalized Natural Protein-Based Polymers as Innovative Biomaterials.Pharmaceutics. 2020 Nov 19;12(11):1115. doi: 10.3390/pharmaceutics12111115. Pharmaceutics. 2020. PMID: 33228250 Free PMC article. Review.
-
Applications of Cyclodextrin-Based Drug Delivery Systems in Inflammation-Related Diseases.Pharmaceutics. 2025 Mar 17;17(3):378. doi: 10.3390/pharmaceutics17030378. Pharmaceutics. 2025. PMID: 40143041 Free PMC article. Review.
-
Recent advances in redox-responsive nanoparticles for combined cancer therapy.Nanoscale Adv. 2022 Jul 28;4(17):3504-3516. doi: 10.1039/d2na00222a. eCollection 2022 Aug 23. Nanoscale Adv. 2022. PMID: 36134355 Free PMC article. Review.
-
Stable triangle: nanomedicine-based synergistic application of phototherapy and immunotherapy for tumor treatment.J Nanobiotechnology. 2024 Oct 18;22(1):635. doi: 10.1186/s12951-024-02925-3. J Nanobiotechnology. 2024. PMID: 39420366 Free PMC article. Review.
References
-
- Perez-Herrero E., Fernandez-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79. - PubMed
-
- Zhuang Y.Y., Deng H.P., Su Y., He L., Wang R.B., Tong G.S. Aptamer-functionalized and backbone redox-responsive hyperbranched polymer for targeted drug delivery in cancer therapy. Biomacromolecules. 2016;17(6):2050–2062. - PubMed
-
- Guan J., Zhou Z.Q., Chen M.H., Li H.Y., Tong D.N., Yao J. Folate-conjugated and pH-responsive polymeric micelles for target-cell-specific anticancer drug delivery. Acta Biomater. 2017;60:244–255. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

