Distinct type 2-high inflammation associated molecular signatures of chronic rhinosinusitis with nasal polyps with comorbid asthma
- PMID: 32637070
- PMCID: PMC7333405
- DOI: 10.1186/s13601-020-00332-z
Distinct type 2-high inflammation associated molecular signatures of chronic rhinosinusitis with nasal polyps with comorbid asthma
Abstract
Background: Patients with chronic rhinosinusitis with nasal polyps (CRSwNP) and comorbid asthma have more severe disease and are difficult to treat. However, the molecular endotypes associated with CRSwNP with comorbid asthma (CRSwNP + AS) are not clear. This study aimed to investigate the characteristics of type 2 inflammation and the molecular signatures associated with CRSwNP + AS.
Methods: A total of 195 subjects; including 65 CRSwNP + AS patients, 99 CRSwNP-alone patients, and 31 healthy control subjects; were enrolled in the study. Nasal tissues from patients with CRSwNP + AS, CRSwNP-alone and control subjects were assessed for infiltration of inflammatory cells and concentrations of total IgE. Whole-transcriptome sequencing was performed and differentially expressed (DE) mRNAs and long non-coding RNAs (lncRNAs) and their associated pathways were analyzed. The correlations between type 2 cytokines and local eosinophils, tissue IgE, and transcriptome signatures were evaluated.
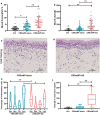
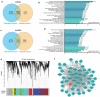
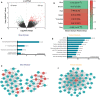
Results: Significantly higher local eosinophil infiltration and higher levels of total IgE were found in nasal tissues from CRSwNP + AS patients than in nasal tissues from CRSwNP-alone patients. Furthermore, atopy and recurrence were significantly more frequent in patients with CRSwNP + AS than in patients with CRSwNP-alone (62.5% vs 28.6% and 66.7% vs 26.9%, respectively). RNA sequencing analysis identified 1988 common DE-mRNAs, and 176 common DE-lncRNAs shared by CRSwNP + AS versus control and CRSwNP-alone versus control. Weighted gene coexpression network analysis (WGCNA) identified LINC01146 as hub lncRNA dysregulated in both subtypes of CRSwNP. Overall, 968 DE-mRNAs and 312 DE-lncRNAs were identified between CRSwNP + AS and CRSwNP-alone. Both pathway enrichment analysis and WGCNA indicated that the phenotypic traits of CRSwNP + AS were mainly associated with higher activities of arachidonic acid metabolism, type 2 cytokines related pathway and fibrinolysis pathway, and lower activity of IL-17 signalling pathway. Furthermore, the expression of type 2 cytokines; IL5 and IL13, was positively correlated with local eosinophil infiltration, tissue IgE level, and the expression of DE-mRNAs that related to arachidonic acid metabolism. Moreover, WGCNA identified HK3-006 as hub lncRNA in yellow module that most positively correlated with phenotypic traits of CRSwNP + AS.
Conclusions: Patients with CRSwNP + AS have distinct type 2-high inflammation-associated molecular signatures in nasal tissues compared to patients with CRSwNP-alone.
Keywords: Asthma; Chronic rhinosinusitis with nasal polyps; Molecular endotype; Transcriptome sequencing; Type 2 inflammation.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no conflicts of interest.
Figures


References
-
- Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(Suppl S29):1–464. - PubMed
-
- Hopkins C. Chronic Rhinosinusitis with nasal polyps. N Engl J Med. 2019;381(1):55–63. - PubMed
-
- Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394:1638–1650. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials

