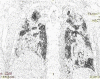A severe refractory COVID-19 patient responding to convalescent plasma; A case series
- PMID: 32637086
- PMCID: PMC7327024
- DOI: 10.1016/j.amsu.2020.06.018
A severe refractory COVID-19 patient responding to convalescent plasma; A case series
Abstract
Introduction: Although some medicines are under research, currently, no specific antiviral drug has been approved to target 2019 novel coronavirus. In this report two severe cases of 2019 novel coronavirus disease (COVID-19) patients have been described who received convalescent plasma (CP).
Case report: Two male cases (a 46-year-old and a 56-year-old) after being diagnosed with severe COVID-19, they deteriorated despite supportive care and antiviral therapy. They started to improve with CP infusion both clinically and radiologically. Finally they were discharged in a very well condition with negative virology tests.
Conclusion: CP might be an effective therapy for severe COVID-19 patients.
Keywords: COVID-19; Convalescent plasma; Coronavirus; SARS-CoV-2.
© 2020 The Authors.
Conflict of interest statement
None to be declared.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous