Spinning disk-remote focusing microscopy
- PMID: 32637230
- PMCID: PMC7316025
- DOI: 10.1364/BOE.389904
Spinning disk-remote focusing microscopy
Abstract
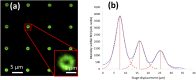
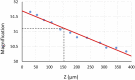
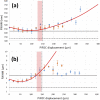
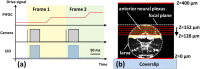
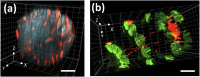
Fast confocal imaging was achieved by combining remote focusing with differential spinning disk optical sectioning to rapidly acquire images of live samples at cellular resolution. Axial and lateral full width half maxima less than 5 µm and 490 nm respectively are demonstrated over 130 µm axial range with a 256 × 128 µm field of view. A water-index calibration slide was used to achieve an alignment that minimises image volume distortion. Application to live biological samples was demonstrated by acquiring image volumes over a 24 µm axial range at 1 volume/s, allowing for the detection of calcium-based neuronal activity in Platynereis dumerilii larvae.
Published by The Optical Society under the terms of the Creative Commons Attribution 4.0 License. Further distribution of this work must maintain attribution to the author(s) and the published article’s title, journal citation, and DOI.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures




References
-
- Botcherby E. J., Juškaitis R., Booth M. J., Wilson T., “An optical technique for remote focusing in microscopy,” Opt. Commun. 281(4), 880–887 (2008).10.1016/j.optcom.2007.10.007 - DOI
LinkOut - more resources
Full Text Sources
