Evaluation of the Anti-Tumor Activity of the Humanized Monoclonal Antibody NEO-201 in Preclinical Models of Ovarian Cancer
- PMID: 32637350
- PMCID: PMC7318110
- DOI: 10.3389/fonc.2020.00805
Evaluation of the Anti-Tumor Activity of the Humanized Monoclonal Antibody NEO-201 in Preclinical Models of Ovarian Cancer
Abstract
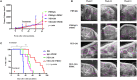
Purpose: Despite high initial response rates with cytoreductive surgery, conventional chemotherapy and the incorporation of biologic agents, ovarian cancer patients often relapse and die from their disease. New approaches are needed to improve patient outcomes. This study was designed to evaluate the antitumor activity of NEO-201 monoclonal antibody (mAb) in preclinical models of ovarian cancer where the NEO-201 target is highly expressed. Experimental Design: Functional analysis of NEO-201 against tumor cell lines was performed by antibody-dependent cellular cytotoxicity (ADCC) assays. Binding of NEO-201 to tumor tissues and cell lines were determined by immunohistochemistry (IHC) and flow cytometry, respectively. Further characterization of the antigen recognized by NEO-201 was performed by mass spectrometry. Ovarian cancer models were used to evaluate the anti-tumor activity of NEO-201 in vivo. NEO-201 at a concentration of 250 g/mouse was injected intraperitoneally (IP) on days 1, 4, and 8. Human PBMCs were injected IP simultaneously as effector cells. Results: Both IHC and flow cytometry revealed that NEO-201 binds prominently to the colon, pancreatic, and mucinous ovarian cancer tissues and cell lines. Immunoprecipitation of the antigen recognized by NEO-201 was performed in human ovarian, colon, and pancreatic cancer cell lines. From these screening, carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) and CEACAM6 were identified as the most likely targets of NEO-201. Our results confirmed that NEO-201 binds different types of cancers; the binding is highly selective for the tumor cells without cross reactivity with the surrounding healthy tissue. Functional analysis revealed that NEO-201 mediates ADCC killing against human ovarian and colorectal carcinoma cell lines in vitro. In addition, NEO-201 inhibited tumor growth in the presence of activated human PBMCs in orthotopic mouse models of both primary and metastatic ovarian cancer. Importantly, NEO-201 prolonged survival of tumor-bearing mice. Conclusions: These data suggested that NEO-201 has an antitumor activity against tumor cells expressing its antigen. Targeting an antigen expressed in tumors, but not in normal tissues, allows patient selection for optimal treatment. These findings strongly indicate that NEO-201 warrants clinical testing as both a novel therapeutic and diagnostic agent for treatment of ovarian carcinomas. A first in human clinical trial evaluating NEO-201 in adults with chemo-resistant solid tumors is ongoing at the NIH clinical Center.
Keywords: antibody-dependent cellular cytotoxicity; carcinoembryonic antigen-related cell adhesion molecule (CEACAM6); carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5); monoclonal antibody; natural killer cell; tumor-associated antigen.
Copyright © 2020 Zeligs, Morelli, David, Neuman, Hernandez, Hewitt, Ozaki, Osei-Tutu, Anderson, Andresson, Das, Lack, Abdelmaksoud, Fantini, Arlen, Tsang and Annunziata.
Figures


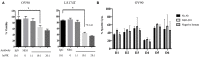
Similar articles
-
Development and Characterization of an Anti-Cancer Monoclonal Antibody for Treatment of Human Carcinomas.Cancers (Basel). 2022 Jun 21;14(13):3037. doi: 10.3390/cancers14133037. Cancers (Basel). 2022. PMID: 35804808 Free PMC article. Review.
-
The Monoclonal Antibody NEO-201 Enhances Natural Killer Cell Cytotoxicity Against Tumor Cells Through Blockade of the Inhibitory CEACAM5/CEACAM1 Immune Checkpoint Pathway.Cancer Biother Radiopharm. 2020 Apr;35(3):190-198. doi: 10.1089/cbr.2019.3141. Epub 2020 Jan 13. Cancer Biother Radiopharm. 2020. PMID: 31928422 Free PMC article.
-
Preclinical Characterization of a Novel Monoclonal Antibody NEO-201 for the Treatment of Human Carcinomas.Front Immunol. 2018 Jan 4;8:1899. doi: 10.3389/fimmu.2017.01899. eCollection 2017. Front Immunol. 2018. PMID: 29354121 Free PMC article.
-
High-grade, chemotherapy-resistant primary ovarian carcinoma cell lines overexpress human trophoblast cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized monoclonal anti-Trop-2 antibody.Gynecol Oncol. 2011 Jul;122(1):171-7. doi: 10.1016/j.ygyno.2011.03.002. Epub 2011 Mar 30. Gynecol Oncol. 2011. PMID: 21453957 Free PMC article.
-
CEACAM6 expression and function in tumor biology: a comprehensive review.Discov Oncol. 2024 May 25;15(1):186. doi: 10.1007/s12672-024-01053-6. Discov Oncol. 2024. PMID: 38796667 Free PMC article. Review.
Cited by
-
First-in-human phase 1 clinical trial of anti-core 1 O-glycans targeting monoclonal antibody NEO-201 in treatment-refractory solid tumors.J Exp Clin Cancer Res. 2023 Mar 29;42(1):76. doi: 10.1186/s13046-023-02649-6. J Exp Clin Cancer Res. 2023. PMID: 36991390 Free PMC article. Clinical Trial.
-
The Protein Landscape of Mucinous Ovarian Cancer: Towards a Theranostic.Cancers (Basel). 2021 Nov 9;13(22):5596. doi: 10.3390/cancers13225596. Cancers (Basel). 2021. PMID: 34830751 Free PMC article. Review.
-
Identification of the O-Glycan Epitope Targeted by the Anti-Human Carcinoma Monoclonal Antibody (mAb) NEO-201.Cancers (Basel). 2022 Oct 12;14(20):4999. doi: 10.3390/cancers14204999. Cancers (Basel). 2022. PMID: 36291783 Free PMC article.
-
Development and Characterization of an Anti-Cancer Monoclonal Antibody for Treatment of Human Carcinomas.Cancers (Basel). 2022 Jun 21;14(13):3037. doi: 10.3390/cancers14133037. Cancers (Basel). 2022. PMID: 35804808 Free PMC article. Review.
-
Harnessing NK cells for cancer immunotherapy: immune checkpoint receptors and chimeric antigen receptors.BMB Rep. 2021 Jan;54(1):44-58. doi: 10.5483/BMBRep.2021.54.1.214. BMB Rep. 2021. PMID: 33298244 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

