Rhinovirus and Innate Immune Function of Airway Epithelium
- PMID: 32637363
- PMCID: PMC7316886
- DOI: 10.3389/fcimb.2020.00277
Rhinovirus and Innate Immune Function of Airway Epithelium
Abstract
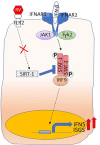
Airway epithelial cells, which lines the respiratory mucosa is in direct contact with the environment. Airway epithelial cells are the primary target for rhinovirus and other inhaled pathogens. In response to rhinovirus infection, airway epithelial cells mount both pro-inflammatory responses and antiviral innate immune responses to clear the virus efficiently. Some of the antiviral responses include the expression of IFNs, endoplasmic reticulum stress induced unfolded protein response and autophagy. Airway epithelial cells also recruits other innate immune cells to establish antiviral state and resolve the inflammation in the lungs. In patients with chronic lung disease, these responses may be either defective or induced in excess leading to deficient clearing of virus and sustained inflammation. In this review, we will discuss the mechanisms underlying antiviral innate immunity and the dysregulation of some of these mechanisms in patients with chronic lung diseases.
Keywords: COPD; ER stress; antiviral responses; asthma; autophagy; dsRNA; rhinovirus.
Copyright © 2020 Ganjian, Rajput, Elzoheiry and Sajjan.
Figures


References
-
- Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., et al. (2008). Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685–701. 10.1083/jcb.200803137 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

