Chimeric antigen receptor signaling: Functional consequences and design implications
- PMID: 32637585
- PMCID: PMC7314561
- DOI: 10.1126/sciadv.aaz3223
Chimeric antigen receptor signaling: Functional consequences and design implications
Abstract
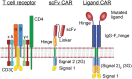
Chimeric antigen receptor (CAR) T cell therapy has transformed the care of refractory B cell malignancies and holds tremendous promise for many aggressive tumors. Despite overwhelming scientific, clinical, and public interest in this rapidly expanding field, fundamental inquiries into CAR T cell mechanistic functioning are still in their infancy. Because CAR T cells are manufactured from donor T lymphocytes, and because CARs incorporate well-characterized T cell signaling components, it has largely been assumed that CARs signal analogously to canonical T cell receptors (TCRs). However, recent studies demonstrate that many aspects of CAR signaling are unique, distinct from endogenous TCR signaling, and potentially even distinct among various CAR constructs. Thus, rigorous and comprehensive proteomic investigations are required for rational engineering of improved CARs. Here, we review what is known about proximal CAR signaling in T cells, compare it to conventional TCR signaling, and outline unmet challenges to improving CAR T cell therapy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Eshhar Z., Waks T., Gross G., Schindler D. G., Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. U.S.A. 90, 720–724 (1993). - PMC - PubMed
-
- Kuwana Y., Asakura Y., Utsunomiya N., Nakanishi M., Arata Y., Itoh S., Nagase F., Kurosawa Y., Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 149, 960–968 (1987). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

