Exploring the nature of the interactions between the molecules of the sodium dodecyl sulfate and water in crystal phases and in the water/vacuum interface
- PMID: 32637679
- PMCID: PMC7327740
- DOI: 10.1016/j.heliyon.2020.e04199
Exploring the nature of the interactions between the molecules of the sodium dodecyl sulfate and water in crystal phases and in the water/vacuum interface
Abstract
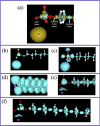
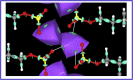
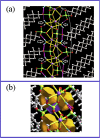
The nature of the interaction between the molecules of the sodium dodecyl sulfate surfactant forming two crystal phases, one anhydrous, NaC12H25O4S and the other, NaC12H25O4S.H2O, hydrated with one water molecule for unit cell, has been studied in detail using the quantum theory of atoms in molecules and a localized electron detector function. It was found that for the anhydrous crystal, the head groups of the surfactant molecules are linked into a head-to-head pattern, by a bond path network of Na-O ionic bonds, where each Na+ atom is attached to four groups. For the hydrated crystal, in addition to these four bonds for Na+, two additional ones appear with the oxygen atoms of the water molecules, forming a bond paths network of ionic Na-O bonds, that link the Na+ atoms with the S groups and the H2O molecules. Each H2O molecule is bonded to two groups via hydrogen bonds, while the groups are linked to a maximum of four Na+ atoms. The phenomenon of aggregation of the sodium dodecyl sulfate molecules at the liquid water/vacuum interface was studied using NVT molecular dynamics simulations. We have found that for surfactant aggregates, the Na+ ions are linked to a maximum of three SO4 - groups and three water molecules that form Na-O bonds. Unlike hydrated crystal, each of the O atoms that make these Na-O bonds is linked to only one Na+ ion. Despite these differences, like the crystal phases, the surfactant molecules tend to form a head-to-head network pattern of ionic Na-O bonds that link their heads. The present results indicate that the clustering of anionic surfactant at the water/vacuum interface is a consequence of the electrostatic alignment of the cationic and anionic groups as occurs in the crystalline phases of sodium dodecyl sulfate.
Keywords: Aggregation of the surfactant molecules; Chemistry; Clustering of the surfactant molecules at the water/vacuum interface; Crystal phases; Materials chemistry; Physical chemistry; Quantum theory of atoms in molecules; Sodium dodecyl sulfonate; Theoretical chemistry.
© 2020 Published by Elsevier Ltd.
Figures







References
-
- Shi L., Tummala N.R., Striolo A. C12E6 and SDS surfactants simulated at the vacuum-water interface. Langmuir. 2010;26:5462–5474. - PubMed
-
- Paredes R., Farinas-Sanchez A.I., Medina-Rodriguez B., Samaniego S., Aray Y., Alvarez L.J. Dynamics of surfactant clustering at interfaces and its influence on the interfacial tension: atomistic simulation of a sodium hexadecane–benzene sulfonate–tetradecane–water system. Langmuir. 2018;34:3146–3157. - PubMed
-
- Hinze W.L. Organized surfactant assemblies in separation science. In: Hinze W.L., Armstrong D., editors. Ordered Media in Chemical Separation. ACS Symposium Series: American Chemical Society; Washington, DC: 1987. pp. 2–82. Chapter 1, 342.
-
- Kinoshita K., Parra E., Needham D. Adsorption of ionic surfactants at microscopic air water interfaces using the Micropipette interfacial area-expansion method: measurement of the diffusion coefficient and renormalization of the mean ionic activity for SDS. J. Colloid Interface Sci. 2017;504:765–779. - PubMed
- Magnus J., Tyrode E. Study of the adsorption of sodium dodecyl sulfate (SDS) at the air/water interface: targeting the sulfate headgroup using vibrational sum frequency spectroscopy. Phys. Chem. Chem. Phys. 2005;7:2635–2640. - PubMed
-
- Watanabe K., Ferrario M., Klein M.L. Molecular dynamics study of a sodium octanoate micelle in aqueous solution. J. Phys. Chem. 1988;92:819–821.
Publication types
LinkOut - more resources
Full Text Sources

